Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs
- PMID: 29254942
- PMCID: PMC5793782
- DOI: 10.1101/gr.222430.117
Transcription factor activity rhythms and tissue-specific chromatin interactions explain circadian gene expression across organs
Abstract
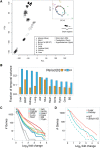
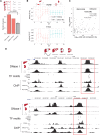
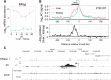
Temporal control of physiology requires the interplay between gene networks involved in daily timekeeping and tissue function across different organs. How the circadian clock interweaves with tissue-specific transcriptional programs is poorly understood. Here, we dissected temporal and tissue-specific regulation at multiple gene regulatory layers by examining mouse tissues with an intact or disrupted clock over time. Integrated analysis uncovered two distinct regulatory modes underlying tissue-specific rhythms: tissue-specific oscillations in transcription factor (TF) activity, which were linked to feeding-fasting cycles in liver and sodium homeostasis in kidney; and colocalized binding of clock and tissue-specific transcription factors at distal enhancers. Chromosome conformation capture (4C-seq) in liver and kidney identified liver-specific chromatin loops that recruited clock-bound enhancers to promoters to regulate liver-specific transcriptional rhythms. Furthermore, this looping was remarkably promoter-specific on the scale of less than 10 kilobases (kb). Enhancers can contact a rhythmic promoter while looping out nearby nonrhythmic alternative promoters, confining rhythmic enhancer activity to specific promoters. These findings suggest that chromatin folding enables the clock to regulate rhythmic transcription of specific promoters to output temporal transcriptional programs tailored to different tissues.
© 2018 Yeung et al.; Published by Cold Spring Harbor Laboratory Press.
Figures



References
-
- Bass J, Lazar MA. 2016. Circadian time signatures of fitness and disease. Science 354: 994–999. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
