In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis
- PMID: 29255022
- PMCID: PMC5776797
- DOI: 10.1073/pnas.1711373114
In vivo inhibition of tryptophan catabolism reorganizes the tuberculoma and augments immune-mediated control of Mycobacterium tuberculosis
Abstract
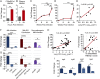
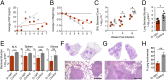
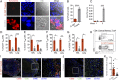
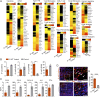
Mycobacterium tuberculosis continues to cause devastating levels of mortality due to tuberculosis (TB). The failure to control TB stems from an incomplete understanding of the highly specialized strategies that M. tuberculosis utilizes to modulate host immunity and thereby persist in host lungs. Here, we show that M. tuberculosis induced the expression of indoleamine 2,3-dioxygenase (IDO), an enzyme involved in tryptophan catabolism, in macrophages and in the lungs of animals (mice and macaque) with active disease. In a macaque model of inhalation TB, suppression of IDO activity reduced bacterial burden, pathology, and clinical signs of TB disease, leading to increased host survival. This increased protection was accompanied by increased lung T cell proliferation, induction of inducible bronchus-associated lymphoid tissue and correlates of bacterial killing, reduced checkpoint signaling, and the relocation of effector T cells to the center of the granulomata. The enhanced killing of M. tuberculosis in macrophages in vivo by CD4+ T cells was also replicated in vitro, in cocultures of macaque macrophages and CD4+ T cells. Collectively, these results suggest that there exists a potential for using IDO inhibition as an effective and clinically relevant host-directed therapy for TB.
Keywords: IDO; T cell; granuloma; macaque; tuberculosis.
Copyright © 2017 the Author(s). Published by PNAS.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- World Health Organization 2016 Global Tuberculosis Report 2016. Available at www.who.int/tb/publications/global_report/gtbr2016_executive_summary.pdf.... Accessed October 16, 2016.
-
- Gandhi NR, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet. 2006;368:1575–1580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI123780/AI/NIAID NIH HHS/United States
- P30 AI050409/AI/NIAID NIH HHS/United States
- UC6 AI058609/AI/NIAID NIH HHS/United States
- R01 AI111914/AI/NIAID NIH HHS/United States
- R21 AI128130/AI/NIAID NIH HHS/United States
- R01 AI123047/AI/NIAID NIH HHS/United States
- R21 AI127222/AI/NIAID NIH HHS/United States
- R01 AI134245/AI/NIAID NIH HHS/United States
- R21 AI127160/AI/NIAID NIH HHS/United States
- P30 GM110760/GM/NIGMS NIH HHS/United States
- R01 AI089323/AI/NIAID NIH HHS/United States
- S10 OD019964/OD/NIH HHS/United States
- R01 AI134240/AI/NIAID NIH HHS/United States
- R21 AI127172/AI/NIAID NIH HHS/United States
- R01 HL106790/HL/NHLBI NIH HHS/United States
- P51 OD011132/OD/NIH HHS/United States
- R01 AI111943/AI/NIAID NIH HHS/United States
- P51 OD011104/OD/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

