Red cell exchange transfusions lower cerebral blood flow and oxygen extraction fraction in pediatric sickle cell anemia
- PMID: 29255068
- PMCID: PMC5833262
- DOI: 10.1182/blood-2017-06-789842
Red cell exchange transfusions lower cerebral blood flow and oxygen extraction fraction in pediatric sickle cell anemia
Abstract
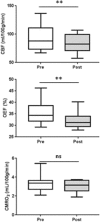
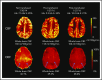
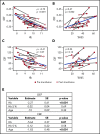
Blood transfusions are the mainstay of stroke prevention in pediatric sickle cell anemia (SCA), but the physiology conferring this benefit is unclear. Cerebral blood flow (CBF) and oxygen extraction fraction (OEF) are elevated in SCA, likely compensating for reduced arterial oxygen content (CaO2). We hypothesized that exchange transfusions would decrease CBF and OEF by increasing CaO2, thereby relieving cerebral oxygen metabolic stress. Twenty-one children with SCA receiving chronic transfusion therapy (CTT) underwent magnetic resonance imaging before and after exchange transfusions. Arterial spin labeling and asymmetric spin echo sequences measured CBF and OEF, respectively, which were compared pre- and posttransfusion. Volumes of tissue with OEF above successive thresholds (36%, 38%, and 40%), as a metric of regional metabolic stress, were compared pre- and posttransfusion. Transfusions increased hemoglobin (Hb; from 9.1 to 10.3 g/dL; P < .001) and decreased Hb S (from 39.7% to 24.3%; P < .001). Transfusions reduced CBF (from 88 to 82.4 mL/100 g per minute; P = .004) and OEF (from 34.4% to 31.2%; P < .001). At all thresholds, transfusions reduced the volume of peak OEF found in the deep white matter, a location at high infarct risk in SCA (P < .001). Reduction of elevated CBF and OEF, both globally and regionally, suggests that CTT mitigates infarct risk in pediatric SCA by relieving cerebral metabolic stress at patient- and tissue-specific levels.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: M.L.H. is a member of the Scientific Advisory Board of the Sickle Cell Transplant Alliance for Research. M.L.H.'s spouse is employed at Pfizer, Inc. The remaining authors declare no competing financial interests.
Figures



Comment in
-
Unwinding the path from anemia to stroke.Blood. 2018 Mar 1;131(9):950-952. doi: 10.1182/blood-2018-01-824185. Blood. 2018. PMID: 29496702 Free PMC article.
References
-
- Earley CJ, Kittner SJ, Feeser BR, et al. . Stroke in children and sickle-cell disease: Baltimore-Washington Cooperative Young Stroke Study. Neurology. 1998;51(1):169-176. - PubMed
-
- Ohene-Frempong K, Weiner SJ, Sleeper LA, et al. . Cerebrovascular accidents in sickle cell disease: rates and risk factors. Blood. 1998;91(1):288-294. - PubMed
-
- Russell MO, Goldberg HI, Hodson A, et al. . Effect of transfusion therapy on arteriographic abnormalities and on recurrence of stroke in sickle cell disease. Blood. 1984;63(1):162-169. - PubMed
-
- Scothorn DJ, Price C, Schwartz D, et al. . Risk of recurrent stroke in children with sickle cell disease receiving blood transfusion therapy for at least five years after initial stroke. J Pediatr. 2002;140(3):348-354. - PubMed
-
- DeBaun MR, Schatz J, Siegel MJ, et al. . Cognitive screening examinations for silent cerebral infarcts in sickle cell disease. Neurology. 1998;50(6):1678-1682. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

