Ligand-activated epidermal growth factor receptor (EGFR) signaling governs endocytic trafficking of unliganded receptor monomers by non-canonical phosphorylation
- PMID: 29255092
- PMCID: PMC5818182
- DOI: 10.1074/jbc.M117.811299
Ligand-activated epidermal growth factor receptor (EGFR) signaling governs endocytic trafficking of unliganded receptor monomers by non-canonical phosphorylation
Abstract
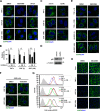
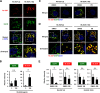
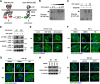
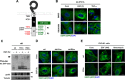
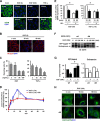
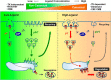
The canonical description of transmembrane receptor function is initial binding of ligand, followed by initiation of intracellular signaling and then internalization en route to degradation or recycling to the cell surface. It is known that low concentrations of extracellular ligand lead to a higher proportion of receptor that is recycled and that non-canonical mechanisms of receptor activation, including phosphorylation by the kinase p38, can induce internalization and recycling. However, no connections have been made between these pathways; i.e. it has yet to be established what happens to unbound receptors following stimulation with ligand. Here we demonstrate that a minimal level of activation of epidermal growth factor receptor (EGFR) tyrosine kinase by low levels of ligand is sufficient to fully activate downstream mitogen-activated protein kinase (MAPK) pathways, with most of the remaining unbound EGFR molecules being efficiently phosphorylated at intracellular serine/threonine residues by activated mitogen-activated protein kinase. This non-canonical, p38-mediated phosphorylation of the C-tail of EGFR, near Ser-1015, induces the clathrin-mediated endocytosis of the unliganded EGFR monomers, which occurs slightly later than the canonical endocytosis of ligand-bound EGFR dimers via tyrosine autophosphorylation. EGFR endocytosed via the non-canonical pathway is largely recycled back to the plasma membrane as functional receptors, whereas p38-independent populations are mainly sorted for lysosomal degradation. Moreover, ligand concentrations balance these endocytic trafficking pathways. These results demonstrate that ligand-activated EGFR signaling controls unliganded receptors through feedback phosphorylation, identifying a dual-mode regulation of the endocytic trafficking dynamics of EGFR.
Keywords: TNF-α; clathrin; endocytosis; epidermal growth factor receptor (EGFR); p38 MAPK; tumor necrosis factor.
© 2018 by The American Society for Biochemistry and Molecular Biology, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures



Similar articles
-
New trend in ligand-induced EGFR trafficking: A dual-mode clathrin-mediated endocytosis model.J Proteomics. 2022 Mar 20;255:104503. doi: 10.1016/j.jprot.2022.104503. Epub 2022 Jan 29. J Proteomics. 2022. PMID: 35093568 Review.
-
Proteomic analysis of the epidermal growth factor receptor (EGFR) interactome and post-translational modifications associated with receptor endocytosis in response to EGF and stress.Mol Cell Proteomics. 2014 Jul;13(7):1644-58. doi: 10.1074/mcp.M114.038596. Epub 2014 May 5. Mol Cell Proteomics. 2014. PMID: 24797263 Free PMC article.
-
Activation of p38 mitogen-activated protein kinase promotes epidermal growth factor receptor internalization.Traffic. 2006 Jun;7(6):686-98. doi: 10.1111/j.1600-0854.2006.00420.x. Traffic. 2006. PMID: 16683917 Free PMC article.
-
Novel mechanism for regulation of epidermal growth factor receptor endocytosis revealed by protein kinase A inhibition.Mol Biol Cell. 2002 May;13(5):1677-93. doi: 10.1091/mbc.01-08-0403. Mol Biol Cell. 2002. PMID: 12006662 Free PMC article.
-
Role of EGF Receptor Regulatory Networks in the Host Response to Viral Infections.Front Cell Infect Microbiol. 2022 Jan 10;11:820355. doi: 10.3389/fcimb.2021.820355. eCollection 2021. Front Cell Infect Microbiol. 2022. PMID: 35083168 Free PMC article. Review.
Cited by
-
HCRP-1 alleviates the malignant phenotype and angiogenesis of oral squamous cell carcinoma cells via the downregulation of the EGFR/STAT3 signaling pathway.Oncol Lett. 2022 Sep 16;24(5):387. doi: 10.3892/ol.2022.13507. eCollection 2022 Nov. Oncol Lett. 2022. PMID: 36276496 Free PMC article.
-
Coordinated ASBT and EGFR Mechanisms for Optimized Liraglutide Nanoformulation Absorption in the GI Tract.Int J Nanomedicine. 2024 Mar 23;19:2973-2992. doi: 10.2147/IJN.S442617. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38544951 Free PMC article.
-
Nuclear epidermal growth factor receptor (nEGFR) in clinical treatment.Heliyon. 2024 Nov 5;10(21):e40150. doi: 10.1016/j.heliyon.2024.e40150. eCollection 2024 Nov 15. Heliyon. 2024. PMID: 39568844 Free PMC article. Review.
-
A Structural Insight Into Two Important ErbB Receptors (EGFR and HER2) and Their Relevance to Non-Small Cell Lung Cancer.Arch Pharm (Weinheim). 2025 Apr;358(4):e2400992. doi: 10.1002/ardp.202400992. Arch Pharm (Weinheim). 2025. PMID: 40194950 Free PMC article. Review.
-
Mechanism of p38 MAPK-induced EGFR endocytosis and its crosstalk with ligand-induced pathways.J Cell Biol. 2021 Jul 5;220(7):e202102005. doi: 10.1083/jcb.202102005. Epub 2021 May 25. J Cell Biol. 2021. PMID: 34032851 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

