Probiotic capsules and xylitol chewing gum to manage symptoms of pharyngitis: a randomized controlled factorial trial
- PMID: 29255098
- PMCID: PMC5738247
- DOI: 10.1503/cmaj.170599
Probiotic capsules and xylitol chewing gum to manage symptoms of pharyngitis: a randomized controlled factorial trial
Abstract
Background: Reducing the use of antibiotics for upper respiratory tract infections is needed to limit the global threat of antibiotic resistance. We estimated the effectiveness of probiotics and xylitol for the management of pharyngitis.
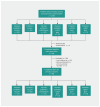
Methods: In this parallel-group factorial randomized controlled trial, participants in primary care (aged 3 years or older) with pharyngitis underwent randomization by nurses who provided sequential intervention packs. Pack contents for 3 kinds of material and advice were previously determined by computer-generated random numbers: no chewing gum, xylitol-based chewing gum (15% xylitol; 5 pieces daily) and sorbitol gum (5 pieces daily). Half of each group were also randomly assigned to receive either probiotic capsules (containing 24 × 109 colony-forming units of lactobacilli and bifidobacteria) or placebo. The primary outcome was mean self-reported severity of sore throat and difficulty swallowing (scale 0-6) in the first 3 days. We used multiple imputation to avoid the assumption that data were missing completely at random.
Results: A total of 1009 individuals consented, 934 completed the baseline assessment, and 689 provided complete data for the primary outcome. Probiotics were not effective in reducing the severity of symptoms: mean severity scores 2.75 with no probiotic and 2.78 with probiotic (adjusted difference -0.001, 95% confidence interval [CI] -0.24 to 0.24). Chewing gum was also ineffective: mean severity scores 2.73 without gum, 2.72 with sorbitol gum (adjusted difference 0.07, 95% CI -0.23 to 0.37) and 2.73 with xylitol gum (adjusted difference 0.01, 95% CI -0.29 to 0.30). None of the secondary outcomes differed significantly between groups, and no harms were reported.
Interpretation: Neither probiotics nor advice to chew xylitol-based chewing gum was effective for managing pharyngitis. Trial registration: ISRCTN, no. ISRCTN51472596.
© 2017 Joule Inc. or its licensors.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sims Sanyahumbi A, Colquhoun S, Wyber R, et al. Global disease burden of group A Streptococcus. In: Ferretti JJ, Stevens DL, Fischetti VA, editors. Streptococcus pyogenes: basic biology to clinical manifestations. Oklahoma City (OK): University of Oklahoma Health Sciences Center; 2016. Available: https://www.ncbi.nlm.nih.gov/books/NBK333415 (accessed 2017 Nov. 17). - PubMed
-
- Little P, Hobbs FD, Moore M, et al. ; PRISM investigators. PRImary care Streptococcal Management (PRISM) study: in vitro study, diagnostic cohorts and a pragmatic adaptive randomised controlled trial with nested qualitative study and cost-effectiveness study. Health Technol Assess 2014;18:vii–xxv, 1–101. - PMC - PubMed
-
- Shulman ST, Bisno AL, Clegg HW, et al. Clinical practice guideline for the diagnosis and management of group A streptococcal pharyngitis: 2012 update by the Infectious Diseases Society of America. Clin Infect Dis 2012;55:1279–82. - PubMed
-
- Harris AM, Hicks LA, Qaseem A; High Value Care Task Force of the American College of Physicians and for the Centers for Disease Control and Prevention. Appropriate antibiotic use for acute respiratory tract infection in adults: advice for high-value care from the American College of Physicians and the Centers for Disease Control and Prevention. Ann Intern Med 2016;164:425–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical