Adaptation of Plasmodium falciparum to its transmission environment
- PMID: 29255304
- PMCID: PMC7116667
- DOI: 10.1038/s41559-017-0419-9
Adaptation of Plasmodium falciparum to its transmission environment
Abstract
Success in eliminating malaria will depend on whether parasite evolution outpaces control efforts. Here, we show that Plasmodium falciparum parasites (the deadliest of the species causing human malaria) found in low-transmission-intensity areas have evolved to invest more in transmission to new hosts (reproduction) and less in within-host replication (growth) than parasites found in high-transmission areas. At the cellular level, this adaptation manifests as increased production of reproductive forms (gametocytes) early in the infection at the expense of processes associated with multiplication inside red blood cells, especially membrane transport and protein trafficking. At the molecular level, this manifests as changes in the expression levels of genes encoding epigenetic and translational machinery. Specifically, expression levels of the gene encoding AP2-G-the transcription factor that initiates reproduction-increase as transmission intensity decreases. This is accompanied by downregulation and upregulation of genes encoding HDAC1 and HDA1-two histone deacetylases that epigenetically regulate the parasite's replicative and reproductive life-stage programmes, respectively. Parasites in reproductive mode show increased reliance on the prokaryotic translation machinery found inside the plastid-derived organelles. Thus, our dissection of the parasite's adaptive regulatory architecture has identified new potential molecular targets for malaria control.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


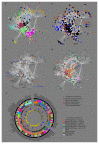
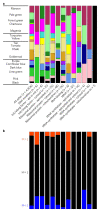
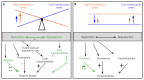
References
-
- Stearns SC. The Evolution of Life Histories. Oxford Univ. Press; New York: 1992.
-
- Gandon S, Mackinnon MJ, Nee S, Read AF. Imperfect vaccines and the evolution of parasite virulence. Nature. 2001;414:751–755. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

