Comparative Analysis of Muscle Hypertrophy Models Reveals Divergent Gene Transcription Profiles and Points to Translational Regulation of Muscle Growth through Increased mTOR Signaling
- PMID: 29255421
- PMCID: PMC5723052
- DOI: 10.3389/fphys.2017.00968
Comparative Analysis of Muscle Hypertrophy Models Reveals Divergent Gene Transcription Profiles and Points to Translational Regulation of Muscle Growth through Increased mTOR Signaling
Abstract
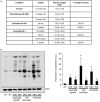
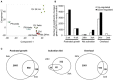
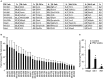
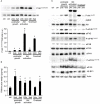
Skeletal muscle mass is a result of the balance between protein breakdown and protein synthesis. It has been shown that multiple conditions of muscle atrophy are characterized by the common regulation of a specific set of genes, termed atrogenes. It is not known whether various models of muscle hypertrophy are similarly regulated by a common transcriptional program. Here, we characterized gene expression changes in three different conditions of muscle growth, examining each condition during acute and chronic phases. Specifically, we compared the transcriptome of Extensor Digitorum Longus (EDL) muscles collected (1) during the rapid phase of postnatal growth at 2 and 4 weeks of age, (2) 24 h or 3 weeks after constitutive activation of AKT, and (3) 24 h or 3 weeks after overload hypertrophy caused by tenotomy of the Tibialis Anterior muscle. We observed an important overlap between significantly regulated genes when comparing each single condition at the two different timepoints. Furthermore, examining the transcriptional changes occurring 24 h after a hypertrophic stimulus, we identify an important role for genes linked to a stress response, despite the absence of muscle damage in the AKT model. However, when we compared all different growth conditions, we did not find a common transcriptional fingerprint. On the other hand, all conditions showed a marked increase in mTORC1 signaling and increased ribosome biogenesis, suggesting that muscle growth is characterized more by translational, than transcriptional regulation.
Keywords: Akt; hypertrophy; immediate early genes; mTORC1; overload; postnatal growth; ribosome biogenesis; skeletal muscle.
Figures

References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

