Effect of Different Doses of Galcanezumab vs Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial
- PMID: 29255900
- PMCID: PMC5838630
- DOI: 10.1001/jamaneurol.2017.3859
Effect of Different Doses of Galcanezumab vs Placebo for Episodic Migraine Prevention: A Randomized Clinical Trial
Erratum in
-
Errors in Text.JAMA Neurol. 2018 Feb 1;75(2):260. doi: 10.1001/jamaneurol.2017.3996. JAMA Neurol. 2018. PMID: 29228075 Free PMC article. No abstract available.
Abstract
Importance: Galcanezumab (LY2951742), a monoclonal antibody against calcitonin gene-related peptide (CGRP), is one of a novel class of new medicines for migraine prevention.
Objective: To assess whether at least 1 dose of galcanezumab was superior to placebo for episodic migraine prevention.
Design, setting, and participants: A randomized clinical trial was conducted in the United States (July 7, 2014, to August 19, 2015) in clinics of 37 licensed physicians with a specialty including, but not limited to, psychiatry, neurology, internal medicine, and primary care. Subcutaneous injections of galcanezumab, 5, 50, 120, or 300 mg, or placebo were given monthly during the 3-month treatment period. A total of 936 patients were assessed; 526 did not meet study entry or baseline criteria and 410 patients were randomly assigned to receive placebo or galcanezumab. Analyses were conducted on an intent-to-treat population, which included all patients who were randomized and received at least 1 dose of study drug.
Interventions: Short-term migraine treatments were allowed as needed except for opioids or barbiturates.
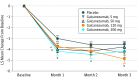
Main outcomes and measures: To determine if at least 1 of the 4 doses of galcanezumab tested was superior to placebo for migraine prevention measured by the mean change from baseline in the number of migraine headache days 9 weeks to 12 weeks after randomization.
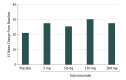
Results: Of the 936 patients assessed, 410 met entry criteria (aged 18-65 years with 4-14 migraine headache days per month and migraine onset prior to age 50 years) and were randomized to receive placebo or galcanezumab. For the primary end point, galcanezumab, 120 mg, significantly reduced migraine headache days compared with placebo (99.6% posterior probability -4.8 days; 90% BCI, -5.4 to -4.2 days vs 95% superiority threshold [Bayesian analysis] -3.7 days; 90% BCI, -4.1 to -3.2 days). Adverse events reported by 5% or more of patients in at least 1 galcanezumab dose group and more frequently than placebo included injection-site pain, upper respiratory tract infection, nasopharyngitis, dysmenorrhea, and nausea.
Conclusions and relevance: Monthly subcutaneous injections of galcanezumab, both 120 mg and 300 mg, demonstrated efficacy (repeated-measures analysis) for the preventive treatment of migraine and support further development in larger phase 3 studies. All dosages were safe and well tolerated for the preventive treatment of episodic migraine.
Trial registration: clinicaltrials.gov Identifier: NCT02163993.
Conflict of interest statement
Figures
References
-
- Global Burden of Disease Study 2013 Collaborators Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386(9995):743-800. - PMC - PubMed
-
- Hepp Z, Dodick DW, Varon SF, Gillard P, Hansen RN, Devine EB. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2015;35(6):478-488. - PubMed
-
- Silberstein SD, Holland S, Freitag F, Dodick DW, Argoff C, Ashman E; Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society . Evidence-based guideline update: pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology. 2012;78(17):1337-1345. - PMC - PubMed
-
- Simpson DM, Hallett M, Ashman EJ, et al. . Practice guideline update summary: botulinum neurotoxin for the treatment of blepharospasm, cervical dystonia, adult spasticity, and headache: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2016;86(19):1818-1826. - PMC - PubMed
-
- Goadsby PJ. Bench to bedside advances in the 21st century for primary headache disorders: migraine treatments for migraine patients. Brain. 2016;139(pt 10):2571-2577. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

