Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond
- PMID: 29257139
- PMCID: PMC5808071
- DOI: 10.1038/leu.2017.329
Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond
Erratum in
-
Correction: Management of relapsed and refractory multiple myeloma: novel agents, antibodies, immunotherapies and beyond.Leukemia. 2019 Apr;33(4):1058-1059. doi: 10.1038/s41375-019-0410-3. Leukemia. 2019. PMID: 30842604 Free PMC article.
Abstract
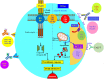
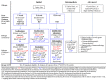
Despite enormous advances, management of multiple myeloma (MM) remains challenging. Multiple factors impact the decision to treat or which regimen to use at MM relapse/progression. Recent major randomized controlled trials (RCTs) showed widely varying progression-free survivals (PFS), ranging from a median of 4 months (MM-003) to 23.6 months (ASPIRE). Based on these RCTs, next-generation proteasome inhibitors (carfilzomib and ixazomib), next-generation immunomodulatory agent (pomalidomide), and monoclonal antibodies (elotuzumab and daratumumab) were approved for relapsed and refractory MM. Daratumumab, targeting CD38, has multiple mechanisms of action including modulation of the immunosuppressive bone marrow micro-environment. In addition to the remarkable single agent activity in refractory MM, daratumumab produced deep responses and superior PFS in MM when combined with lenalidomide/dexamethasone, or bortezomib/dexamethasone. Other anti-CD38 antibodies, such as isatuximab and MOR202, are undergoing assessment. Elotuzumab, targeting SLAMF7, yielded superior response rates and PFS when combined with lenalidomide/dexamethasone. New combinations of these next generation novel agents and/or antibodies are undergoing clinical trials. Venetoclax, an oral BH3 mimetic inhibiting BCL2, showed single agent activity in MM with t(11;14), and is being studied in combination with bortezomib/dexamethasone. Selinexor, an Exportin-1 inhibitor, yielded promising results in quad- or penta-refractory MM including patients resistant to daratumumab. Pembrolizumab, an anti-PD1 check-point inhibitor, is being tested in combination with lenalidomide/dexamethasone or pomalidomide/dexamethasone. Chimeric antigen receptor-T cells targeting B-cell maturation antigen have yielded deep responses in RRMM. Finally, salvage autologous stem cell transplantation (ASCT) remains an important treatment in MM relapsing/progressing after a first ASCT. Herein, the clinical trial data of these agents are summarized, cautious interpretation of RCTs highlighted, and algorithm for salvage treatment of relapse/refractory MM proposed.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Palumbo A, Anderson K. Multiple myeloma. N Engl J Med 2011; 364: 1046–1060. - PubMed
-
- Harousseau J-L, Attal M, Avet-Loiseau H. The role of complete response in multiple myeloma. Blood 2009; 114: 3139–3146. - PubMed
-
- Zent C, Wilson C, Tricot G, Jagannath S, Siegel D, Desikan K et al. Oligoclonal protein bands and Ig isotype switching in multiple myeloma treated with high-dose therapy and hematopoietic cell transplantation. Blood 1998; 91: 3518–3523. - PubMed
-
- Chim C, Lie A, Chan E, Leung Y, Cheung S, Chan S et al. A staged approach with vincristine, adriamycin, and dexamethasone followed by bortezomib, thalidomide, and dexamethasone before autologous hematopoietic stem cell transplantation in the treatment of newly diagnosed multiple myeloma. Ann Hematol 2010; 89: 1019–1027. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

