Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila
- PMID: 29258011
- PMCID: PMC5981003
- DOI: 10.1016/j.conb.2017.12.002
Do the right thing: neural network mechanisms of memory formation, expression and update in Drosophila
Abstract
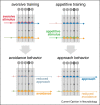
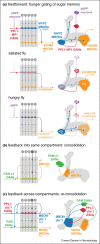
When animals learn, plasticity in brain networks that respond to specific cues results in a change in the behavior that these cues elicit. Individual network components in the mushroom bodies of the fruit fly Drosophila melanogaster represent cues, learning signals and behavioral outcomes of learned experience. Recent findings have highlighted the importance of dopamine-driven plasticity and activity in feedback and feedforward connections, between various elements of the mushroom body neural network. These computational motifs have been shown to be crucial for long term olfactory memory consolidation, integration of internal states, re-evaluation and updating of learned information. The often recurrent circuit anatomy and a prolonged requirement for activity in parts of these underlying networks, suggest that self-sustained and precisely timed activity is a fundamental feature of network computations in the insect brain. Together these processes allow flies to continuously adjust the content of their learned knowledge and direct their behavior in a way that best represents learned expectations and serves their most pressing current needs.
Copyright © 2017 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Heisenberg M., Borst A., Wagner S., Byers D. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2:1–30. - PubMed
-
- Tanaka N.K., Tanimoto H., Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508:711–755. - PubMed
-
- Waddell S., Armstrong J.D., Kitamoto T., Kaiser K., Quinn W.G. The amnesiac gene product is expressed in two neurons in the Drosophila brain that are critical for memory. Cell. 2000;103:805–813. - PubMed
-
- Takemura S., Aso Y., Hige T., Wong A., Lu Z., Xu C.S., Rivlin P.K., Hess H.F., Zhao T., Parag T. A connectome of a learning and memory center in the adult Drosophila brain. eLife. 2017;6:e26975. - PMC - PubMed
-
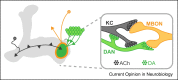
Traces the α lobe neuropil of the mushroom body from focused ion-beam milling scanning electron microscopy data. Synaptic morphologies reconstructed at the microstructural level reveal unexpected novel classes such as DAN to MBON and KC to DAN direct synapses. Imaging experiments also show that DAN to MBON synapses are functional and induce a slow depolarization in the postsynaptic MBON.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

