Effect of Prophylactic Indomethacin in Extremely Low Birth Weight Infants Based on the Predicted Risk of Severe Intraventricular Hemorrhage
- PMID: 29258076
- PMCID: PMC6282184
- DOI: 10.1159/000485172
Effect of Prophylactic Indomethacin in Extremely Low Birth Weight Infants Based on the Predicted Risk of Severe Intraventricular Hemorrhage
Abstract
Background: Prophylactic indomethacin reduces the risk of severe intraventricular hemorrhage (IVH) but does not reduce death or neurodevelopmental impairment (NDI) among extremely low birth weight (ELBW) infants. Some investigators have suggested that prophylactic indomethacin may have a greater treatment effect on severe IVH among infants at high risk for severe IVH.
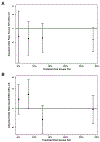
Objective: To determine whether the relative treatment effects of prophylactic indomethacin on severe IVH and the composite outcome of death or NDI vary based on the risk of severe IVH.
Methods: Post hoc analysis of the Trial of Indomethacin Prophylaxis in Preterms (TIPP). We generated a model to predict the risk for severe IVH based on gestational age, birth weight, antenatal steroids, delivery mode, outborn status, sex, and 5-min Apgar score, and we divided the TIPP participants into risk quartiles. We used logistic regression to determine the adjusted odds ratios (aOR) of severe IVH and death or NDI based on indomethacin treatment for each quartile.
Results: The relative treatment effects of prophylactic indomethacin on severe IVH did not vary based on the predicted risk of severe IVH: quartile 1: aOR 0.68 (95% confidence interval [CI] 0.19-2.37); quartile 2: aOR 0.61 (95% CI 0.27-1.42); quartile 3: aOR 0.63 (95% CI 0.31-1.31); quartile 4: aOR 0.58 (95% CI 0.32-1.05). The relative treatment effect of prophylactic indomethacin on death or NDI did not vary significantly between quartiles.
Conclusions: These findings do not support selective prophylactic indomethacin treatment to improve long-term outcomes of ELBW infants at high risk for severe IVH.
Keywords: Brain injury; Extremely low birth weight infants; Indomethacin; Neurodevelopmental disability.
© 2017 S. Karger AG, Basel.
Conflict of interest statement
Conflicts of Interest
The authors have no conflicts of interest to disclose.
Figures
References
-
- Schmidt B, Asztalos EV, Roberts RS, Robertson CMT, Suave RS, Whitfield MF; Trial of Indomethacin Prophylaxis in Preterms (TIPP) Investigators. Impact of bronchopulmonary dysplasia, brain injury, and severe retinopathy on the outcome of extremely low-birth-weight infants at 18 months: results from the trial of indomethacin prophylaxis in preterms. JAMA 2003; 289:1124–9. - PubMed
-
- Schmidt B, Davis P, Moddemann D Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL; Trial of Indomethacin Prophylaxis in Preterms Investigators. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 2001; 344:1966–72. - PubMed
-
- Luque MJ, Tapia JL, Villarroel L, Marshall G, Musante G, Carlo W, Kattan J, Neocosur Neonatal Network. A risk prediction model for severe intraventricular hemorrhage in very low birth weight infants and the effect of prophylactic indomethacin. J Perinatol 2014; 34:43–8. - PubMed
-
- Lea CL, Smith-Collins A, Luyt K. Protecting the premature brain: current evidence-based strategies for minimising perinatal brain injury in preterm infants. Arch Dis Child Fetal Neonatal Ed 2017; 102:F176–82. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

