Role of Galectins in Multiple Myeloma
- PMID: 29258207
- PMCID: PMC5751341
- DOI: 10.3390/ijms18122740
Role of Galectins in Multiple Myeloma
Abstract
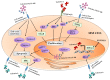
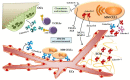
Galectins are a family of lectins that bind β-galactose-containing glycoconjugates and are characterized by carbohydrate-recognition domains (CRDs). Galectins exploit several biological functions, including angiogenesis, regulation of immune cell activities and cell adhesion, in both physiological and pathological processes, as tumor progression. Multiple myeloma (MM) is a plasma cell (PC) malignancy characterized by the tight adhesion between tumoral PCs and bone marrow (BM) microenvironment, leading to the increase of PC survival and drug resistance, MM-induced neo-angiogenesis, immunosuppression and osteolytic bone lesions. In this review, we explore the expression profiles and the roles of galectin-1, galectin-3, galectin-8 and galectin-9 in the pathophysiology of MM. We focus on the role of these lectins in the interplay between MM and BM microenvironment cells showing their involvement in MM progression mainly through the regulation of PC survival and MM-induced angiogenesis and osteoclastogenesis. The translational impact of these pre-clinical pieces of evidence is supported by recent data that indicate galectins could be new attractive targets to block MM cell growth in vivo and by the evidence that the expression levels of LGALS1 and LGALS8, genes encoding for galectin-1 and galectin-8 respectively, correlate to MM patients' survival.
Keywords: galectin-1; galectin-3; galectin-8; galectin-9; galectins; myeloma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Role of Galectins in Tumors and in Clinical Immunotherapy.Int J Mol Sci. 2018 Feb 1;19(2):430. doi: 10.3390/ijms19020430. Int J Mol Sci. 2018. PMID: 29389859 Free PMC article. Review.
-
Mesenchymal stem cell-mediated T cell suppression occurs through secreted galectins.Int J Oncol. 2011 Feb;38(2):385-90. doi: 10.3892/ijo.2010.869. Epub 2010 Dec 9. Int J Oncol. 2011. PMID: 21165555
-
Galectin-loaded cells as a platform for the profiling of lectin specificity by fluorescent neoglycoconjugates: a case study on galectins-1 and -3 and the impact of assay setting.Glycobiology. 2008 Apr;18(4):315-24. doi: 10.1093/glycob/cwn009. Epub 2008 Feb 6. Glycobiology. 2008. PMID: 18256179
-
Thermodynamic binding studies of galectin-1, -3 and -7.Glycoconj J. 2002;19(7-9):459-65. doi: 10.1023/B:GLYC.0000014075.62724.d0. Glycoconj J. 2002. PMID: 14758069 Review.
-
Lactulose as a novel template for anticancer drug development targeting galectins.Chem Biol Drug Des. 2018 Oct;92(4):1801-1808. doi: 10.1111/cbdd.13348. Epub 2018 Jul 1. Chem Biol Drug Des. 2018. PMID: 29888844
Cited by
-
The complexity of tumour angiogenesis based on recently described molecules.Contemp Oncol (Pozn). 2021;25(1):33-44. doi: 10.5114/wo.2021.105075. Epub 2021 Apr 15. Contemp Oncol (Pozn). 2021. PMID: 33911980 Free PMC article. Review.
-
Classification of Solitary Plasmacytoma, Is it more Intricate than Presently Suggested? A Commentary.J Cancer. 2018 Oct 10;9(21):3894-3897. doi: 10.7150/jca.26854. eCollection 2018. J Cancer. 2018. PMID: 30410592 Free PMC article. Review.
-
Treatment of B-cell precursor acute lymphoblastic leukemia with the Galectin-1 inhibitor PTX008.J Exp Clin Cancer Res. 2018 Mar 27;37(1):67. doi: 10.1186/s13046-018-0721-7. J Exp Clin Cancer Res. 2018. PMID: 29580262 Free PMC article.
-
Galectin-1 and Galectin-3 in B-Cell Precursor Acute Lymphoblastic Leukemia.Int J Mol Sci. 2022 Nov 18;23(22):14359. doi: 10.3390/ijms232214359. Int J Mol Sci. 2022. PMID: 36430839 Free PMC article.
-
Galectin Family Members: Emerging Novel Targets for Lymphoma Therapy?Front Oncol. 2022 May 23;12:889034. doi: 10.3389/fonc.2022.889034. eCollection 2022. Front Oncol. 2022. PMID: 35677161 Free PMC article. Review.
References
-
- Cummings R.D., Liu F.T., Vasta G.R. Galectins. In: Varki A., Cummings R.D., Esko J.D., Stanley P., Hart G.W., Aebi M., Darvill A.G., Kinoshita T., Packer N.H., Prestegard J.H., et al., editors. Essentials of Glycobiology. 3rd ed. Cold Spring Harbor; New York, NY, USA: 2015. - PubMed
-
- Ahmad N., Gabius H.J., Andre S., Kaltner H., Sabesan S., Roy R., Liu B., Macaluso F., Brewer C.F. Galectin-3 precipitates as a pentamer with synthetic multivalent carbohydrates and forms heterogeneous cross-linked complexes. J. Biol. Chem. 2004;279:10841–10847. doi: 10.1074/jbc.M312834200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

