Evaluation of a field-deployable reverse transcription-insulated isothermal PCR for rapid and sensitive on-site detection of Zika virus
- PMID: 29258444
- PMCID: PMC5735522
- DOI: 10.1186/s12879-017-2852-4
Evaluation of a field-deployable reverse transcription-insulated isothermal PCR for rapid and sensitive on-site detection of Zika virus
Abstract
Background: The recent emergence of Zika virus (ZIKV) in Brazil and its precipitous expansion throughout the Americas has highlighted the urgent need for a rapid and reliable on-site diagnostic assay suitable for viral detection. Such point-of-need (PON), low-cost diagnostics are essential for ZIKV control in vulnerable areas with limited resources.
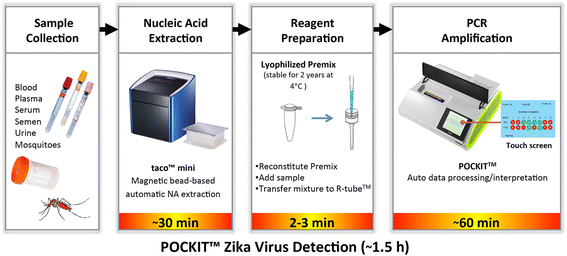
Methods: We developed and evaluated a ZIKV-specific field-deployable RT-iiPCR reagent set targeting the E gene for rapid detection of ZIKV in ZIKV-spiked human and mosquito specimens, and compared its performance to the Center for Disease Control and Prevention (CDC) and Pan American Health Organization (PAHO) RT-qPCR assays targeting the E and NS2B genes, respectively.
Results: These assays demonstrated exclusive specificity for ZIKV (African and Asian lineages), had limits of detection ranging from 10 to 100 in vitro transcribed RNA copies/μl and detection endpoints at 10 plaque forming units/ml of infectious tissue culture fluid. Analysis of human whole blood, plasma, serum, semen, urine, and mosquito pool samples spiked with ZIKV showed an agreement of 90% (k = 0.80), 92% (k = 0.82), 95% (k = 0.86), 92% (k = 0.81), 90% (k = 0.79), and 100% (k = 1), respectively, between the RT-iiPCR assay and composite results from the reference RT-qPCR assays. Overall, the concurrence between the ZIKV RT-iiPCR and the reference RT-qPCR assays was 92% (k = 0.83).
Conclusions: The ZIKV RT-iiPCR has a performance comparable to the reference CDC and PAHO RT-qPCR assays but provides much faster results (~1.5 h) with a field-deployable system that can be utilized as a PON diagnostic with the potential to significantly improve the quality of the health care system in vulnerable areas.
Keywords: Insulated isothermal PCR; POCKIT; Point-of-need assay; Zika virus; iiPCR.
Conflict of interest statement
Ethics approval and consent to participate
Kentucky Blood Center provided the routine donor blood samples collected from allogeneic blood donors for the purpose of donor testing. The donor consent for blood donation includes that the blood sample(s) collected could be used for research purposes. Furthermore, the Institutional Review Board (IRB) designee determined that this project does not require IRB review because the samples utilized in this study were either commercial or de-identified from the Center for Clinical and Translational Science Biorepository (BioBank).
Consent for publication
All the authors approved the final version of the manuscript.
Competing interests
MC, YL, DW, AS, RFC, GB, and UBRB declare no competing interests. CT, PAL, PC, HGC, and HTW are affiliated with GeneReach USA, Lexington, MA. However, this does not alter our adherence to BMC Infectious Diseases policies on sharing data and materials.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
Validation of the Pockit Dengue Virus Reagent Set for Rapid Detection of Dengue Virus in Human Serum on a Field-Deployable PCR System.J Clin Microbiol. 2018 Apr 25;56(5):e01865-17. doi: 10.1128/JCM.01865-17. Print 2018 May. J Clin Microbiol. 2018. PMID: 29436418 Free PMC article.
-
Improved detection of Zika virus RNA in human and animal specimens by a novel, highly sensitive and specific real-time RT-PCR assay targeting the 5'-untranslated region of Zika virus.Trop Med Int Health. 2017 May;22(5):594-603. doi: 10.1111/tmi.12857. Epub 2017 Mar 16. Trop Med Int Health. 2017. PMID: 28214373
-
One-step RT-qPCR assay for ZIKV RNA detection in Aedes aegypti samples: a protocol to study infection and gene expression during ZIKV infection.Parasit Vectors. 2020 Mar 14;13(1):128. doi: 10.1186/s13071-020-4002-x. Parasit Vectors. 2020. PMID: 32171303 Free PMC article.
-
Loop-Mediated Isothermal Amplification (LAMP) for the Diagnosis of Zika Virus: A Review.Viruses. 2019 Dec 23;12(1):19. doi: 10.3390/v12010019. Viruses. 2019. PMID: 31877989 Free PMC article. Review.
-
Zika virus diagnosis: challenges and solutions.Clin Microbiol Infect. 2019 Feb;25(2):142-146. doi: 10.1016/j.cmi.2018.12.002. Epub 2018 Dec 12. Clin Microbiol Infect. 2019. PMID: 30553031 Review.
Cited by
-
On-site detection system of Candidatus Liberibacter asiaticus by using TaqMan probe-based insulated isothermal polymerase chain reaction (iiPCR).PLoS One. 2023 Jun 23;18(6):e0287699. doi: 10.1371/journal.pone.0287699. eCollection 2023. PLoS One. 2023. PMID: 37352328 Free PMC article.
-
Mouse-Adapted SARS-CoV-2 MA10 Strain Displays Differential Pulmonary Tropism and Accelerated Viral Replication, Neurodissemination, and Pulmonary Host Responses in K18-hACE2 Mice.mSphere. 2023 Feb 21;8(1):e0055822. doi: 10.1128/msphere.00558-22. Epub 2023 Feb 2. mSphere. 2023. PMID: 36728430 Free PMC article.
-
Validation of the Pockit Dengue Virus Reagent Set for Rapid Detection of Dengue Virus in Human Serum on a Field-Deployable PCR System.J Clin Microbiol. 2018 Apr 25;56(5):e01865-17. doi: 10.1128/JCM.01865-17. Print 2018 May. J Clin Microbiol. 2018. PMID: 29436418 Free PMC article.
-
Evaluation of a Sample-to-Result POCKIT Central SARS-CoV-2 PCR System.Diagnostics (Basel). 2023 Jun 29;13(13):2219. doi: 10.3390/diagnostics13132219. Diagnostics (Basel). 2023. PMID: 37443612 Free PMC article.
-
An RT-PCR panel for rapid serotyping of dengue virus serotypes 1 to 4 in human serum and mosquito on a field-deployable PCR system.PLoS One. 2019 Mar 25;14(3):e0214328. doi: 10.1371/journal.pone.0214328. eCollection 2019. PLoS One. 2019. PMID: 30908535 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

