Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome
- PMID: 29258545
- PMCID: PMC5735803
- DOI: 10.1186/s12974-017-1004-5
Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome
Erratum in
-
Correction to: Dendrimer-mediated delivery of N-acetyl cysteine to microglia in a mouse model of Rett syndrome.J Neuroinflammation. 2018 Jan 12;15(1):14. doi: 10.1186/s12974-018-1056-1. J Neuroinflammation. 2018. PMID: 29329583 Free PMC article.
Abstract
Background: Rett syndrome (RTT) is a pervasive developmental disorder that is progressive and has no effective cure. Immune dysregulation, oxidative stress, and excess glutamate in the brain mediated by glial dysfunction have been implicated in the pathogenesis and worsening of symptoms of RTT. In this study, we investigated a new nanotherapeutic approach to target glia for attenuation of brain inflammation/injury both in vitro and in vivo using a Mecp2-null mouse model of Rett syndrome.
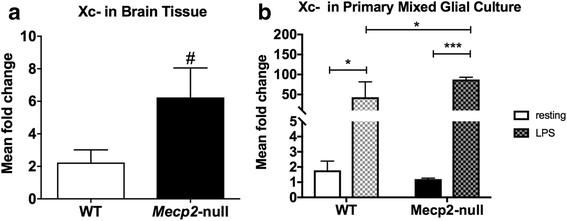
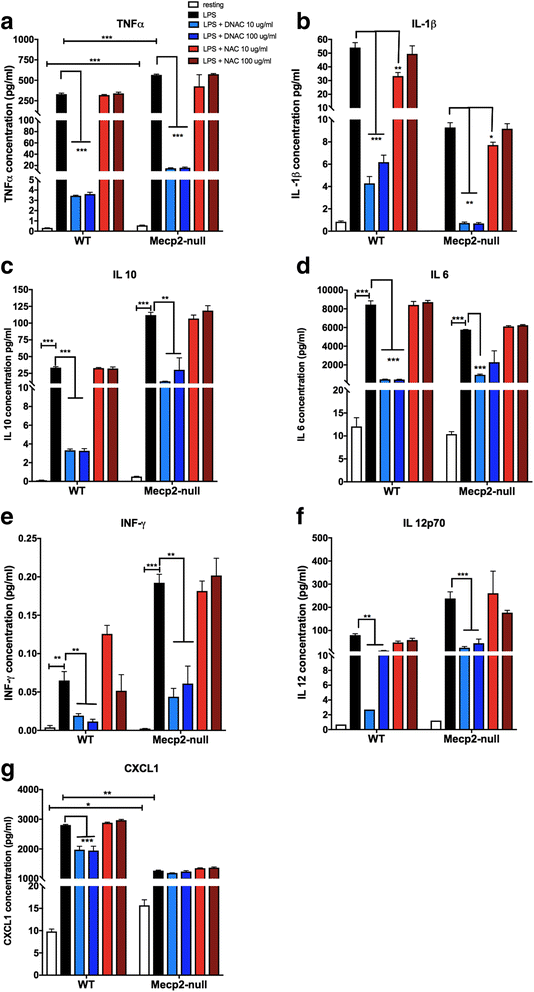
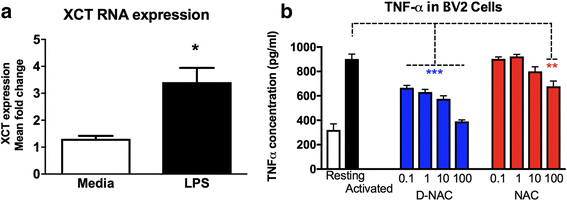
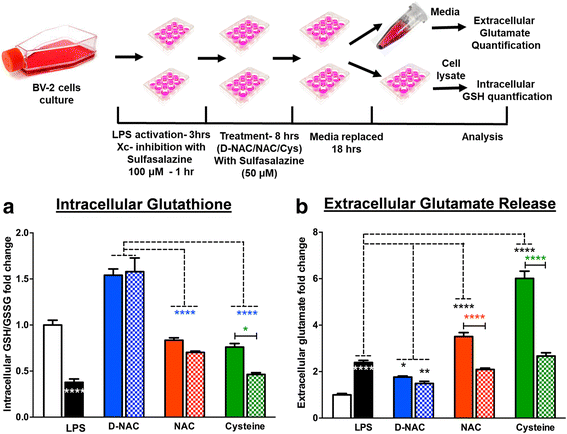
Methods: To determine whether inflammation and immune dysregulation were potential targets for dendrimer-based therapeutics in RTT, we assessed the immune response of primary glial cells from Mecp2-null and wild-type (WT) mice to LPS. Using dendrimers that intrinsically target activated microglia and astrocytes, we studied N-acetyl cysteine (NAC) and dendrimer-conjugated N-acetyl cysteine (D-NAC) effects on inflammatory cytokines by PCR and multiplex assay in WT vs Mecp2-null glia. Since the cysteine-glutamate antiporter (Xc-) is upregulated in Mecp2-null glia when compared to WT, the role of Xc- in the uptake of NAC and L-cysteine into the cell was compared to that of D-NAC using BV2 cells in vitro. We then assessed the ability of D-NAC given systemically twice weekly to Mecp2-null mice to improve behavioral phenotype and lifespan.
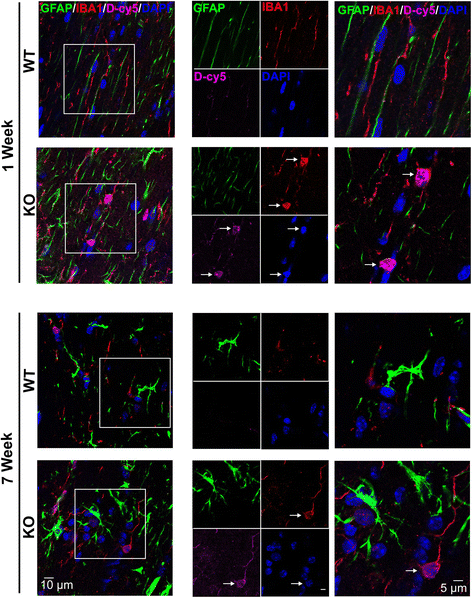
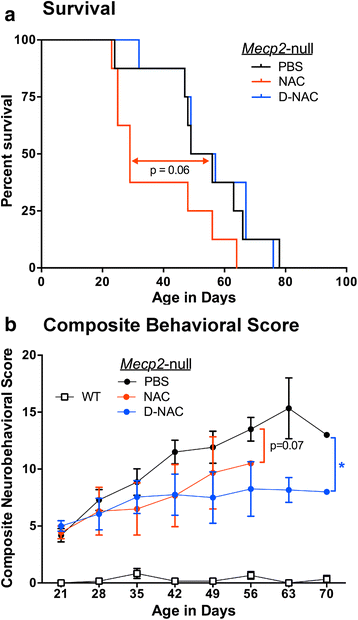
Results: We demonstrated that the mixed glia derived from Mecp2-null mice have an exaggerated inflammatory and oxidative stress response to LPS stimulation when compared to WT glia. Expression of Xc- was significantly upregulated in the Mecp2-null glia when compared to WT and was further increased in the presence of LPS stimulation. Unlike NAC, D-NAC bypasses the Xc- for cell uptake, increasing intracellular GSH levels while preventing extracellular glutamate release and excitotoxicity. Systemically administered dendrimers were localized in microglia in Mecp2-null mice, but not in age-matched WT littermates. Treatment with D-NAC significantly improved behavioral outcomes in Mecp2-null mice, but not survival.
Conclusions: These results suggest that delivery of drugs using dendrimer nanodevices offers a potential strategy for targeting glia and modulating oxidative stress and immune responses in RTT.
Keywords: Glutamate; Mecp2-null; Microglia; N-Acetyl cysteine; PAMAM dendrimer; Rett syndrome; System Xc−.
Conflict of interest statement
Ethics approval
Animal experiments were conducted within the guidelines set forth by the National Institutes of Health (NIH) and the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. All animal protocols were approved by the Johns Hopkins Animal Care and Use Committee.
Consent for publication
Not applicable.
Competing interests
SK and RK hold a patent on dendrimer-based nanodevices for therapeutic and imaging purposes (US8889101) and are founders of a company focused on clinical translation of this compound.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

