Amplification of Oncolytic Vaccinia Virus Widespread Tumor Cell Killing by Sunitinib through Multiple Mechanisms
- PMID: 29259007
- PMCID: PMC6501576
- DOI: 10.1158/0008-5472.CAN-15-3308
Amplification of Oncolytic Vaccinia Virus Widespread Tumor Cell Killing by Sunitinib through Multiple Mechanisms
Abstract
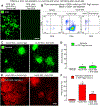
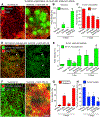
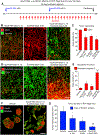
Oncolytic viruses pose many questions in their use in cancer therapy. In this study, we assessed the potential of mpJX-594 (mouse-prototype JX-594), a replication-competent vaccinia virus administered by intravenous injection, to target the tumor vasculature, produce immune activation and tumor cell killing more widespread than the infection, and suppress invasion and metastasis. These actions were examined in RIP-Tag2 transgenic mice with pancreatic neuroendocrine tumors that developed spontaneously and progressed as in humans. mpJX-594 initially infected tumor vascular endothelial cells, leading to vascular pruning and prolonged leakage in tumors but not in normal organs; parallel effects were observed in U87 gliomas. Viral infection spread to tumor cells, where tumor cell killing was much more widespread than the infection. Widespread tumor cell killing at 5 days was prevented by depletion of CD8+ T lymphocytes and did not require GM-CSF, as mpJX-594 variants that expressed human, mouse, or no GM-CSF produced equivalent amounts of killing. The antivascular, antitumor, and antimetastatic effects of mpJX-594 were amplified by concurrent or sequential administration of sunitinib, a multitargeted receptor tyrosine kinase inhibitor. These effects were not mimicked by selective inhibition of VEGFR2 despite equivalent vascular pruning, but were accompanied by suppression of regulatory T cells and greater influx of activated CD8+ T cells. Together, our results showed that mpJX-594 targets tumor blood vessels, spreads secondarily to tumor cells, and produces widespread CD8+ T-cell-dependent tumor cell killing in primary tumors and metastases, and that these effects can be amplified by coadministration of sunitinib.Significance: These findings reveal multiple unrecognized features of the antitumor properties of oncolytic vaccinia viruses, all of which can be amplified by the multitargeted kinase inhibitor sunitinib. Cancer Res; 78(4); 922-37. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Figures




References
-
- Lawler SE, Speranza MC, Cho CF, Chiocca EA. Oncolytic Viruses in Cancer Treatment: A Review. JAMA Oncol 2017;3:841–49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

