ER Stress Signaling Promotes the Survival of Cancer "Persister Cells" Tolerant to EGFR Tyrosine Kinase Inhibitors
- PMID: 29259014
- PMCID: PMC5815936
- DOI: 10.1158/0008-5472.CAN-17-1904
ER Stress Signaling Promotes the Survival of Cancer "Persister Cells" Tolerant to EGFR Tyrosine Kinase Inhibitors
Abstract
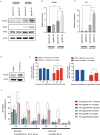
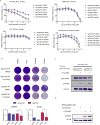
An increasingly recognized component of resistance to tyrosine kinase inhibitors (TKI) involves persistence of a drug-tolerant subpopulation of cancer cells that survive despite effective eradication of the majority of the cell population. Multiple groups have demonstrated that these drug-tolerant persister cells undergo transcriptional adaptation via an epigenetic state change that promotes cell survival. Because this mode of TKI drug tolerance appears to involve transcriptional addiction to specific genes and pathways, we hypothesized that systematic functional screening of EGFR TKI/transcriptional inhibitor combination therapy would yield important mechanistic insights and alternative drug escape pathways. We therefore performed a genome-wide CRISPR/Cas9 enhancer/suppressor screen in EGFR-dependent lung cancer PC9 cells treated with erlotinib + THZ1 (CDK7/12 inhibitor) combination therapy, a combination previously shown to suppress drug-tolerant cells in this setting. As expected, suppression of multiple genes associated with transcriptional complexes (EP300, CREBBP, and MED1) enhanced erlotinib/THZ1 synergy. Unexpectedly, we uncovered nearly every component of the recently described ufmylation pathway in the synergy suppressor group. Loss of ufmylation did not affect canonical downstream EGFR signaling. Instead, absence of this pathway triggered a protective unfolded protein response associated with STING upregulation, promoting protumorigenic inflammatory signaling but also unique dependence on Bcl-xL. These data reveal that dysregulation of ufmylation and ER stress comprise a previously unrecognized TKI drug tolerance pathway that engages survival signaling, with potentially important therapeutic implications.Significance: These findings reveal a novel function of the recently described ufmylation pathway, an ER stress survival signaling in drug-tolerant persister cells, which has important biological and therapeutic implications. Cancer Res; 78(4); 1044-57. ©2017 AACR.
©2017 American Association for Cancer Research.
Conflict of interest statement
Conflict of Interest Statement: D.A.B. is a consultant for N-of-One.
Figures




References
-
- Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nature reviews Cancer. 2013;13:714–26. - PubMed
-
- Lee HJ, Zhuang G, Cao Y, Du P, Kim HJ, Settleman J. Drug resistance via feedback activation of Stat3 in oncogene-addicted cancer cells. Cancer cell. 2014;26:207–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

