Trophoblast lineage specification, differentiation and their regulation by oxygen tension
- PMID: 29259074
- PMCID: PMC5741095
- DOI: 10.1530/JOE-17-0402
Trophoblast lineage specification, differentiation and their regulation by oxygen tension
Abstract
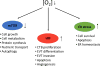
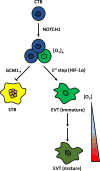
Development of the early embryo takes place under low oxygen tension. Under such conditions, the embryo implants and the trophectoderm, the outer layer of blastocyst, proliferate, forming the cytotrophoblastic shell, the early placenta. The cytotrophoblasts (CTBs) are the so-called epithelial 'stem cells' of the placenta, which, depending on the signals they receive, can differentiate into either extravillous trophoblast (EVT) or syncytiotrophoblast (STB). EVTs anchor the placenta to the uterine wall and remodel maternal spiral arterioles in order to provide ample blood supply to the growing fetus. STBs arise through CTB fusion, secrete hormones necessary for pregnancy maintenance and form a barrier across which nutrient and gas exchange can take place. The bulk of EVT differentiation occurs during the first trimester, before the onset of maternal arterial blood flow into the intervillous space of the placenta, and thus under low oxygen tension. These conditions affect numerous signaling pathways, including those acting through hypoxia-inducible factor, the nutrient sensor mTOR and the endoplasmic reticulum stress-induced unfolded protein response pathway. These pathways are known to be involved in placental development and disease, and specific components have even been identified as directly involved in lineage-specific trophoblast differentiation. Nevertheless, much controversy surrounds the role of hypoxia in trophoblast differentiation, particularly with EVT. This review summarizes previous studies on this topic, with the intent of integrating these results and synthesizing conclusions that resolve some of the controversy, but then also pointing to remaining areas, which require further investigation.
Keywords: cytotrophoblast; extravillous trophoblast; hypoxia; hypoxia-inducible factor; placenta; syncytiotrophoblast.
© 2018 Society for Endocrinology.
Conflict of interest statement
There are no conflicts of interest to report.
Figures


References
-
- Alsat E, Wyplosz P, Malassiné A, Guibourdenche J, Porquet D, Nessmann C, Evain-Brion D. Hypoxia impairs cell fusion and differentiation process in human cytotrophoblast, in vitro. J Cell Physiol. 1996;168:346–353. - PubMed
-
- Arsham AM, Howell JJ, Simon MC. A novel hypoxia-inducible factor-independent hypoxic response regulating mammalian target of rapamycin and its targets. J Biol Chem. 2003;278:29655–29660. - PubMed
-
- Baczyk D, Drewlo S, Proctor L, Dunk C, Lye S, Kingdom J. Glial cell missing-1 transcription factor is required for the differentiation of the human trophoblast. Cell Death Differentiation. 2009;16:719–727. - PubMed
-
- Baczyk D, Dunk C, Huppertz B, Maxwell C, Reister F, Giannoulias D, Kingdom JC. Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta. 2006;27:367–374. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

