NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans
- PMID: 29259175
- PMCID: PMC5736597
- DOI: 10.1038/s41598-017-17649-8
NLRP3 inflammasome is a key player in human vulvovaginal disease caused by Candida albicans
Abstract
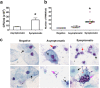
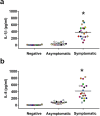
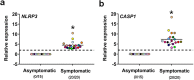
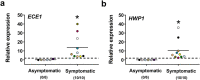
The expression of host inflammatory and Candida albicans putative virulence factors was studied in women with vulvovaginal candidiasis (VVC; twenty) or colonized by the fungus but asymptomatic (carriers; fifteen) or non-colonized asymptomatic (ten subjects). Overexpression of genes encoding NLRP3 and caspase-1 inflammasome components sharply differentiated VVC patients from asymptomatic colonized or non-colonized women. Inflammasome expression was coupled with neutrophils recruitment in the vagina of VVC women and IL-1β and IL-8 production. Both cytokines were present, though to a lower concentration, also in the vaginal fluid of colonized and non-colonized women. Secretory aspartyl proteinases (SAPs) and hyphae associated genes HWP1 and ECE1 were upregulated in VVC but with some differences among infected women. The most overexpressed SAP gene was SAP2, that correlated with neutrophils accumulation. Our data provide clinical evidence that the intracytoplasmic activation of NLRP3 inflammasome complex plays a critical, pathogenesis-relevant role in human VVC.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

