Sixteen isostructural phosphonate metal-organic frameworks with controlled Lewis acidity and chemical stability for asymmetric catalysis
- PMID: 29259195
- PMCID: PMC5736719
- DOI: 10.1038/s41467-017-02335-0
Sixteen isostructural phosphonate metal-organic frameworks with controlled Lewis acidity and chemical stability for asymmetric catalysis
Abstract
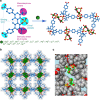
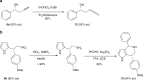
Heterogeneous catalysts typically lack the specific steric control and rational electronic tuning required for precise asymmetric catalysis. Here we demonstrate that a phosphonate metal-organic framework (MOF) platform that is robust enough to accommodate up to 16 different metal clusters, allowing for systematic tuning of Lewis acidity, catalytic activity and enantioselectivity. A total of 16 chiral porous MOFs, with the framework formula [M3 L 2(solvent)2] that have the same channel structures but different surface-isolated Lewis acid metal sites, are prepared from a single phosphono-carboxylate ligand of 1,1'-biphenol and 16 different metal ions. The phosphonate MOFs possessing tert-butyl-coated channels exhibited high thermal stability and good tolerances to boiling water, weak acid and base. The MOFs provide a versatile family of heterogeneous catalysts for asymmetric allylboration, propargylation, Friedel-Crafts alkylation and sulfoxidation with good to high enantioselectivity. In contrast, the homogeneous catalyst systems cannot catalyze the test reactions enantioselectively.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Blaser HU, Federsel HJ. Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions. Weinheim: Wiley-VCH; 2004.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

