Ubiquitin Ligase Huwe1 Modulates Spermatogenesis by Regulating Spermatogonial Differentiation and Entry into Meiosis
- PMID: 29259204
- PMCID: PMC5736635
- DOI: 10.1038/s41598-017-17902-0
Ubiquitin Ligase Huwe1 Modulates Spermatogenesis by Regulating Spermatogonial Differentiation and Entry into Meiosis
Abstract
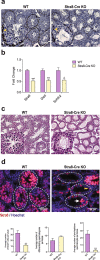
Spermatogenesis consists of a series of highly regulated processes that include mitotic proliferation, meiosis and cellular remodeling. Although alterations in gene expression are well known to modulate spermatogenesis, posttranscriptional mechanisms are less well defined. The ubiquitin proteasome system plays a significant role in protein turnover and may be involved in these posttranscriptional mechanisms. We previously identified ubiquitin ligase Huwe1 in the testis and showed that it can ubiquitinate histones. Since modulation of histones is important at many steps in spermatogenesis, we performed a complete characterization of the functions of Huwe1 in this process by examining the effects of its inactivation in the differentiating spermatogonia, spermatocytes and spermatids. Inactivation of Huwe1 in differentiating spermatogonia led to their depletion and formation of fewer pre-leptotene spermatocytes. The cell degeneration was associated with an accumulation of DNA damage response protein γH2AX, impaired downstream signalling and apoptosis. Inactivation of Huwe1 in spermatocytes indicated that Huwe1 is not essential for meiosis and spermiogenesis, but can result in accumulation of γH2AX. Collectively, these results provide a comprehensive survey of the functions of Huwe1 in spermatogenesis and reveal Huwe1's critical role as a modulator of the DNA damage response pathway in the earliest steps of spermatogonial differentiation.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

