Films of Bacteria at Interfaces (FBI): Remodeling of Fluid Interfaces by Pseudomonas aeruginosa
- PMID: 29259206
- PMCID: PMC5736630
- DOI: 10.1038/s41598-017-17721-3
Films of Bacteria at Interfaces (FBI): Remodeling of Fluid Interfaces by Pseudomonas aeruginosa
Erratum in
-
Author Correction: Films of Bacteria at Interfaces (FBI): Remodeling of Fluid Interfaces by Pseudomonas aeruginosa.Sci Rep. 2019 Nov 25;9(1):17809. doi: 10.1038/s41598-019-54303-x. Sci Rep. 2019. PMID: 31767957 Free PMC article.
Abstract
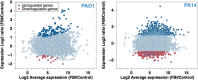
Bacteria at fluid interfaces endure physical and chemical stresses unique to these highly asymmetric environments. The responses of Pseudomonas aeruginosa PAO1 and PA14 to a hexadecane-water interface are compared. PAO1 cells form elastic films of bacteria, excreted polysaccharides and proteins, whereas PA14 cells move actively without forming an elastic film. Studies of PAO1 mutants show that, unlike solid-supported biofilms, elastic interfacial film formation occurs in the absence of flagella, pili, or certain polysaccharides. Highly induced genes identified in transcriptional profiling include those for putative enzymes and a carbohydrate metabolism enzyme, alkB2; this latter gene is not upregulated in PA14 cells. Notably, PAO1 mutants lacking the alkB2 gene fail to form an elastic layer. Rather, they form an active film like that formed by PA14. These findings demonstrate that genetic expression is altered by interfacial confinement, and suggest that the ability to metabolize alkanes may play a role in elastic film formation at oil-water interfaces.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
-
- Paradh, A. Gram-negative spoilage bacteria in brewing. Brewing Microbiology: Managing Microbes, Ensuring Quality and Valorising Waste, 175 (Woodhead Publishing, 2015).
-
- Wilking JN, Angelini TE, Seminara A, Brenner MP, Weitz DA. Biofilms as complex fluids. MRS bulletin. 2011;36:385–391. doi: 10.1557/mrs.2011.71. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

