Oocyte aging underlies female reproductive aging: biological mechanisms and therapeutic strategies
- PMID: 29259413
- PMCID: PMC5715832
- DOI: 10.1007/s12522-015-0209-5
Oocyte aging underlies female reproductive aging: biological mechanisms and therapeutic strategies
Abstract
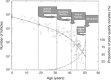
In recent years, postponement of marriage and childbearing in women of reproductive age has led to an increase in the incidence of age-related infertility. The reproductive aging process in women is assumed to occur due to a decrease in both the quantity and quality of the oocytes, with the ultimate result being a decline in fecundity. This age-related decline in fecundity is strongly dependent on oocyte quality, which is critical for fertilization and subsequent embryo development. Aged oocytes display increased chromosomal abnormality and dysfunction of cellular organelles, both of which factor into oocyte quality. In particular, mitochondrial dysfunction has been suggested as a major contributor to the reduction in oocyte quality as well as to chromosomal abnormalities in aged oocytes and embryos. Participation of oxidative stress in the oocyte aging process has been proposed because oxidative stress has the capacity to induce mitochondrial dysfunction and directly damage many intracellular components of the oocytes such as lipids, protein, and DNA. In an attempt to improve mitochondrial function in aged oocytes, several therapeutic strategies have been investigated using both animal models and assisted reproductive technology. Here, we review the biological mechanisms and present status of therapeutic strategies in the female reproductive aging field and indicate possible future therapeutic strategies.
Keywords: Endoplasmic reticulum; Mitochondria; Oocyte aging; Oxidative stress; Reproductive aging.
Figures


References
-
- Velde ER, Pearson PL. The variability of female reproductive ageing. Hum Reprod Update, 2002, 8, 141–154 10.1093/humupd/8.2.141 - DOI - PubMed
-
- Broekmans FJ, Knauff EA, Velde ER, Macklon NS, Fauser BC. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol Metab, 2007, 18, 58–65 10.1016/j.tem.2007.01.004 - DOI - PubMed
-
- Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Steirteghem A, Cohen J, Crosignani PG, Devroey P et al. Fertility and ageing. Hum Reprod Update, 2005, 11, 261–276 10.1093/humupd/dmi006 - DOI - PubMed
-
- Dougall K, Beyene Y, Nachtigall RD. Age shock: misperceptions of the impact of age on fertility before and after IVF in women who conceived after age 40. Hum Reprod, 2013, 28, 350–356 10.1093/humrep/des409 - DOI - PMC - PubMed
-
- Takeshima K, Saito H, Nakaza A, Kuwahara A, Ishihara O, Irahara M, Hirahara H, Yoshimura Y, Sakumoto T. Efficacy, safety, and trends in assisted reproductive technology in Japan—analysis of four‐year data from the national registry system. J Assist Reprod Genet, 2014, 31, 477–484396946710.1007/s10815‐014‐0181‐8 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
