Regulatory Considerations for Gene Therapy Products in the US, EU, and Japan
- PMID: 29259533
- PMCID: PMC5733859
Regulatory Considerations for Gene Therapy Products in the US, EU, and Japan
Abstract
Developers of gene therapy products (GTPs) must adhere to additional regulation beyond that of traditional small-molecule therapeutics, due to the unique mechanism-of-action of GTPs and the subsequent novel risks arisen. We have provided herein a summary of the regulatory structure under which GTPs fall in the United States, the European Union, and Japan, and a comprehensive overview of the regulatory guidance applicable to the developer of GTP. Understanding the regulatory requirements for seeking GTP market approval in these major jurisdictions is crucial for an effective and expedient path to market. The novel challenges facing GTP developers is highlighted by a case study of alipogene tiparvovec (Glybera).
Keywords: ATMP; EMA; FDA; Japan; MHLW; advanced therapeutic medicinal product; gene therapy; regulation.
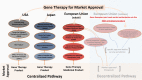
Figures

References
-
- National Human Genome Research Institute [Internet]. The Cost of Sequencing the Human Genome. Available from: https://www.genome.gov/sequencingcosts/
-
- Abbott A. Human genome at ten: the human race. Nature. 2010;464:668–9. - PubMed
-
- Juod SW. Celebrating a Milestone: FDA’s Approval of First Genetically-Engineered Product. 2007. [Cited 3 July 2017] Available from: https://www.fda.gov/aboutfda/whatwedo/history/productregulation/selectio...
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
