Pathophysiology and therapeutic potential of cardiac fibrosis
- PMID: 29259712
- PMCID: PMC5725925
- DOI: 10.1186/s41232-017-0046-5
Pathophysiology and therapeutic potential of cardiac fibrosis
Abstract
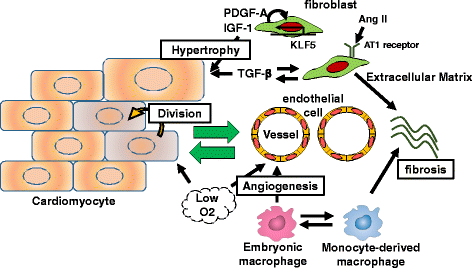
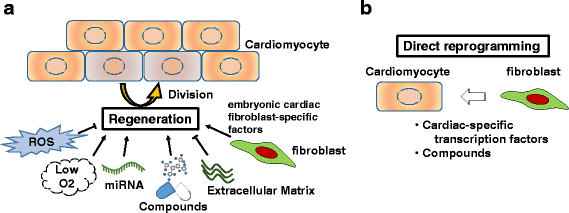
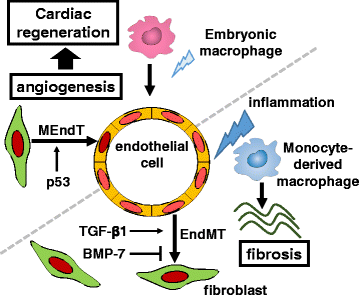
Inflammatory and fibrotic responses to myocardial damage are essential for cardiac repair; however, these responses often result in extensive fibrotic remodeling with impaired systolic function. Recent reports have suggested that such acute phase responses provide a favorable environment for endogenous cardiac regeneration, which is mainly driven by the division of pre-existing cardiomyocytes (CMs). Existing CMs in mammals can re-acquire proliferative activity after substantial cardiac damage, and elements other than CMs in the physiological and/or pathological environment, such as hypoxia, angiogenesis, and the polarity of infiltrating macrophages, have been reported to regulate replication. Cardiac fibroblasts comprise the largest cell population in terms of cell number in the myocardium, and they play crucial roles in the proliferation and protection of CMs. The in vivo direct reprogramming of functional CMs has been investigated in cardiac regeneration. Currently, growth factors, transcription factors, microRNAs, and small molecules promoting the regeneration and protection of these CMs have also been actively researched. Here, we summarize and discuss current studies on the relationship between cardiac inflammation and fibrosis, and cardiac regeneration and protection, which would be useful for the development of therapeutic strategies to treat and prevent advanced heart failure.
Keywords: Angiogenesis; Cardiac fibroblasts; Cardiac regeneration; Cardiomyocytes; Direct reprogramming.
Figures
Similar articles
-
Cardiac fibrosis in myocardial infarction-from repair and remodeling to regeneration.Cell Tissue Res. 2016 Sep;365(3):563-81. doi: 10.1007/s00441-016-2431-9. Epub 2016 Jun 21. Cell Tissue Res. 2016. PMID: 27324127 Free PMC article. Review.
-
Cardiac regeneration with pluripotent stem cell-derived cardiomyocytes and direct cardiac reprogramming.Regen Ther. 2019 Jun 27;11:95-100. doi: 10.1016/j.reth.2019.06.004. eCollection 2019 Dec. Regen Ther. 2019. PMID: 31304202 Free PMC article. Review.
-
Progress and Challenge of Cardiac Regeneration to Treat Heart Failure.J Cardiol. 2019 Feb;73(2):97-101. doi: 10.1016/j.jjcc.2018.10.002. Epub 2018 Nov 9. J Cardiol. 2019. PMID: 30420106 Review.
-
Cardiac fibrosis: Cell biological mechanisms, molecular pathways and therapeutic opportunities.Mol Aspects Med. 2019 Feb;65:70-99. doi: 10.1016/j.mam.2018.07.001. Epub 2018 Aug 2. Mol Aspects Med. 2019. PMID: 30056242 Review.
-
Direct cellular reprogramming for cardiac repair and regeneration.Eur J Heart Fail. 2016 Feb;18(2):145-56. doi: 10.1002/ejhf.446. Epub 2015 Dec 3. Eur J Heart Fail. 2016. PMID: 26635186 Review.
Cited by
-
Characteristics and prognostic value of right ventricular (dys)function in patients with non-ischaemic dilated cardiomyopathy assessed with cardiac magnetic resonance imaging.ESC Heart Fail. 2021 Apr;8(2):1055-1063. doi: 10.1002/ehf2.13072. Epub 2021 Feb 9. ESC Heart Fail. 2021. PMID: 33560582 Free PMC article.
-
The regenerative response of cardiac interstitial cells.J Mol Cell Biol. 2023 Mar 29;14(10):mjac059. doi: 10.1093/jmcb/mjac059. J Mol Cell Biol. 2023. PMID: 36271843 Free PMC article.
-
The effects of cardiac stretch on atrial fibroblasts: analysis of the evidence and potential role in atrial fibrillation.Cardiovasc Res. 2022 Jan 29;118(2):440-460. doi: 10.1093/cvr/cvab035. Cardiovasc Res. 2022. PMID: 33576384 Free PMC article. Review.
-
Modified mRNA-Mediated CCN5 Gene Transfer Ameliorates Cardiac Dysfunction and Fibrosis without Adverse Structural Remodeling.Int J Mol Sci. 2024 Jun 6;25(11):6262. doi: 10.3390/ijms25116262. Int J Mol Sci. 2024. PMID: 38892449 Free PMC article.
-
Molecular Interplay in Cardiac Fibrosis: Exploring the Functions of RUNX2, BMP2, and Notch.Rev Cardiovasc Med. 2024 Oct 16;25(10):368. doi: 10.31083/j.rcm2510368. eCollection 2024 Oct. Rev Cardiovasc Med. 2024. PMID: 39484128 Free PMC article. Review.
References
-
- Banerjee I, Fuseler JJW, Price RL, Borg TK, Baudino T, Jw F, et al. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am Physiol. 2007;293:1883–91. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

