Resident fibroblasts in the kidney: a major driver of fibrosis and inflammation
- PMID: 29259716
- PMCID: PMC5725902
- DOI: 10.1186/s41232-017-0048-3
Resident fibroblasts in the kidney: a major driver of fibrosis and inflammation
Abstract
Background: Chronic kidney disease (CKD) is a leading cause of end stage renal disease (ESRD) and cardiovascular morbidity and mortality worldwide, resulting in a growing social and economic burden. The prevalence and burden of CKD is anticipated to further increase over the next decades as a result of aging.
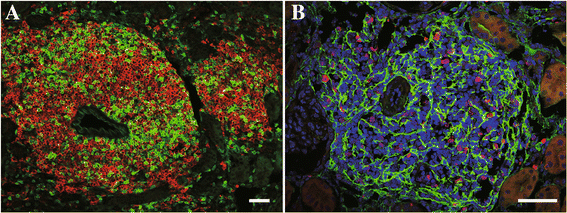
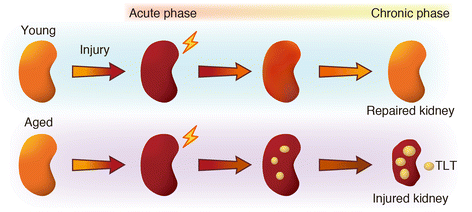
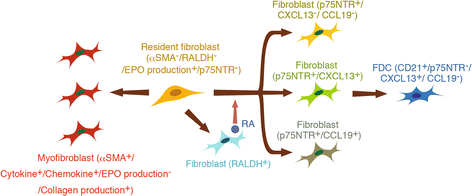
Main body of abstract: In the pathogenesis of CKD, irrespective of the etiology, resident fibroblasts are key players and have been demonstrated to play crucial roles for disease initiation and progression. In response to injury, resident fibroblasts transdifferentiate into myofibroblasts that express alpha smooth muscle actin (αSMA) and have an increased capacity to produce large amounts of extracellular matrix (ECM) proteins, leading to renal fibrosis. In addition to this fundamental role of fibroblasts as drivers for renal fibrosis, growing amounts of evidence have shown that resident fibroblasts are also actively involved in initiating and promoting inflammation during kidney injury. During the myofibroblastic transition described above, resident fibroblasts activate NF-κB signaling and produce pro-inflammatory cytokines and chemokines, promoting inflammation. Furthermore, under aging milieu, resident fibroblasts transdifferentiate into several distinct phenotypic fibroblasts, including CXCL13/CCL19-producing fibroblasts, retinoic acid-producing fibroblasts, and follicular dendritic cells, in response to injury and orchestrate tertiary lymphoid tissue (TLT) formation, which results in uncontrolled aberrant inflammation and retards tissue repair. Anti-inflammatory agents can improve myofibroblastic transdifferentiation and abolish TLT formation, suggesting that targeting these inflammatory fibroblasts can potentially ameliorate kidney disease.
Short conclusion: Beyond its conventional role as an executor of fibrosis, resident fibroblasts display more pro-inflammatory phenotypes and contribute actively to driving inflammation during kidney injury.
Keywords: CXCL13; Chronic kidney disease; Erythropoietin; Fibroblast; Heterogeneity; Inflammation; Myofibroblast; Tertiary lymphoid tissue.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

