Effects of CTLA4-Ig on human monocytes
- PMID: 29259723
- PMCID: PMC5725919
- DOI: 10.1186/s41232-017-0054-5
Effects of CTLA4-Ig on human monocytes
Abstract
Background: Abatacept, a CTLA4-Ig fusion protein attenuates T cell activation by inhibiting the CD80/86-CD28 costimulatory pathway that is required for the proper T cell activation and thus displays beneficial effects in the treatment of rheumatoid arthritis (RA). Although some studies have disclosed the in vitro effects of this biological agent on the immune-competent cells, the precise mechanisms of action in RA still remain unclear. The current studies were therefore undertaken to explore the effects of abatacept on monocytes in detail.
Methods: Monocytes from healthy donors were cultured in the presence of staphylococcal enterotoxin B (SEB) with pharmacologically attainable concentrations of abatacept or control IgG-Fc. The expression of CD80 and CD86 and the induction of apoptosis of monocytes were measured by flow cytometry. The expression of CD80 and CD86 messenger RNA (mRNA) was determined by quantitative RT-PCR.
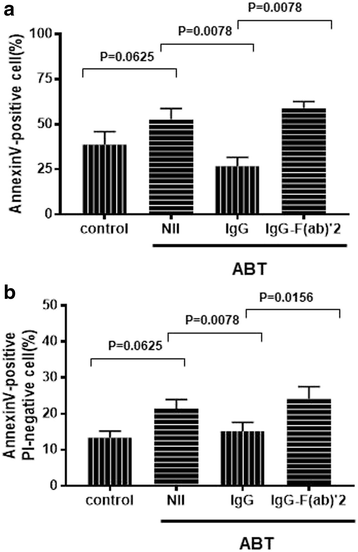
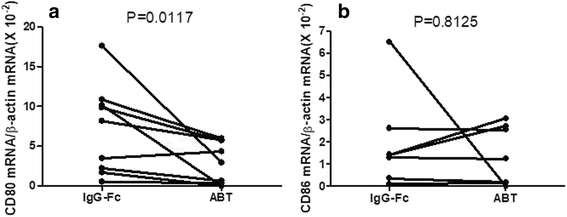
Results: Abatacept promoted apoptosis of SEB-stimulated monocytes. The induction of apoptosis of monocytes by these biological agents was reversed by the addition of IgG, but not IgG-F(ab')2 fragments. Furthermore, abatacept significantly suppressed the expression of CD80, but not that of CD86 at protein levels. Finally, abatacept significantly suppressed the expression of mRNA for CD80 of monocytes stimulated with SEB, but not that of CD86.
Conclusions: These results demonstrate that one of the mechanisms of action of abatacept involves the induction of apoptosis of monocytes, which involves interaction with Fc receptor on monocytes. Moreover, the data also demonstrate that abatacept selectively suppresses the expression of CD80 at mRNA levels.
Keywords: Abatacept; Apoptosis; Costimulation molecules; Monocytes.
Conflict of interest statement
Peripheral blood mononuclear cells (PBMCs) were obtained from healthy adult volunteers who gave informed consent. All procedures were approved by the ethics committee in the Kitasato University School of Medicine.Not applicable.The authors declare that they have no competing interests.Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Mitoma H, Horiuchi T, Tsukamoto H, Tamimoto Y, Kimoto Y, Uchino A, et al. Mechanisms for cytotoxic effects of anti-tumor necrosis factor agents on transmembrane tumor necrosis factor alpha-expressing cells: comparison among infliximab, etanercept, and adalimumab. Arthritis Rheum. 2008;58:1248–1257. doi: 10.1002/art.23447. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

