Discovery of a Slow Tight Binding LPA1 Antagonist (ONO-0300302) for the Treatment of Benign Prostatic Hyperplasia
- PMID: 29259748
- PMCID: PMC5733272
- DOI: 10.1021/acsmedchemlett.7b00383
Discovery of a Slow Tight Binding LPA1 Antagonist (ONO-0300302) for the Treatment of Benign Prostatic Hyperplasia
Abstract
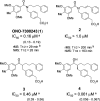
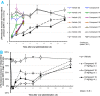
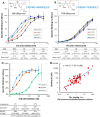
Scaffold hopping from the amide group of lead compound ONO-7300243 (1) to a secondary alcohol successfully gave a novel chemotype lysophosphatidic acid receptor 1 (LPA1) antagonist 4. Wash-out experiments using rat isolated urethra showed that compound 4 possesses a tight binding feature to the LPA1 receptor. Further modification of two phenyl groups of 1 to pyrrole and an indane moiety afforded an optimized compound ONO-0300302 (19). Despite its high i.v. clearance, 19 inhibited significantly an LPA-induced increase of intraurethral pressure (IUP) in rat (3 mg/kg, p.o.) and dog (1 mg/kg, p.o.) over 12 h. Binding experiments with [3H]-ONO-0300302 suggest that the observed long duration action is because of the slow tight binding character of 19.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Terakado M.; Suzuki H.; Hashimura K.; Tanaka M.; Ueda H.; Kohno H.; Fujimoto T.; Saga H.; Nakade S.; Habashita H.; Takaoka Y.; Seko T. Discovery of ONO-7300243 from a novel class of lysophosphatidic acid receptor 1 antagonists: from hit to lead. ACS Med. Chem. Lett. 2016, 7, 913–918. 10.1021/acsmedchemlett.6b00225. - DOI - PMC - PubMed
-
- Ohta H.; Sato K.; Murata N.; Damirin A.; Malchinkhuu E.; Kon J.; Kimura T.; Tobo M.; Yamazaki Y.; Watanabe T.; Yagi M.; Sato M.; Suzuki R.; Murooka H.; Sakai T.; Nishitoba T.; Im D. S.; Nochi H.; Tamoto K.; Tomura H.; Okajima F. Ki16425, a subtype-selective antagonist for EDG-family lysophosphatidic acid receptors. Mol. Pharmacol. 2003, 64, 994–1005. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

