mTOR-Dependent Cell Proliferation in the Brain
- PMID: 29259984
- PMCID: PMC5702949
- DOI: 10.1155/2017/7082696
mTOR-Dependent Cell Proliferation in the Brain
Abstract
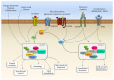
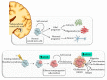
The mammalian Target of Rapamycin (mTOR) is a molecular complex equipped with kinase activity which controls cell viability being key in the PI3K/PTEN/Akt pathway. mTOR acts by integrating a number of environmental stimuli to regulate cell growth, proliferation, autophagy, and protein synthesis. These effects are based on the modulation of different metabolic pathways. Upregulation of mTOR associates with various pathological conditions, such as obesity, neurodegeneration, and brain tumors. This is the case of high-grade gliomas with a high propensity to proliferation and tissue invasion. Glioblastoma Multiforme (GBM) is a WHO grade IV malignant, aggressive, and lethal glioma. To date, a few treatments are available although the outcome of GBM patients remains poor. Experimental and pathological findings suggest that mTOR upregulation plays a major role in determining an aggressive phenotype, thus determining relapse and chemoresistance. Among several activities, mTOR-induced autophagy suppression is key in GBM malignancy. In this article, we discuss recent evidence about mTOR signaling and its role in normal brain development and pathological conditions, with a special emphasis on its role in GBM.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

