Characterization of a Feline Influenza A(H7N2) Virus
- PMID: 29260686
- PMCID: PMC5749472
- DOI: 10.3201/eid2401.171240
Characterization of a Feline Influenza A(H7N2) Virus
Abstract
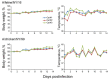
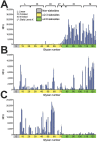
During December 2016-February 2017, influenza A viruses of the H7N2 subtype infected ≈500 cats in animal shelters in New York, NY, USA, indicating virus transmission among cats. A veterinarian who treated the animals also became infected with feline influenza A(H7N2) virus and experienced respiratory symptoms. To understand the pathogenicity and transmissibility of these feline H7N2 viruses in mammals, we characterized them in vitro and in vivo. Feline H7N2 subtype viruses replicated in the respiratory organs of mice, ferrets, and cats without causing severe lesions. Direct contact transmission of feline H7N2 subtype viruses was detected in ferrets and cats; in cats, exposed animals were also infected via respiratory droplet transmission. These results suggest that the feline H7N2 subtype viruses could spread among cats and also infect humans. Outbreaks of the feline H7N2 viruses could, therefore, pose a risk to public health.
Keywords: H7N2; New York; United States; feline; influenza; influenza virus; respiratory infections; viruses; zoonoses; zoonotic infection.
Figures





References
-
- Wright PF, Neumann G, Kawaoka Y. Orthomyxoviruses. In: Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, et al., editors. Fields Virology. Sixth ed. Philadelphia, PA, USA: Lippincott Williams & Wilkins; 2013. p. 1186–243.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

