An Easy-Made, Economical and Efficient Carbon-Doped Amorphous TiO₂ Photocatalyst Obtained by Microwave Assisted Synthesis for the Degradation of Rhodamine B
- PMID: 29261118
- PMCID: PMC5744382
- DOI: 10.3390/ma10121447
An Easy-Made, Economical and Efficient Carbon-Doped Amorphous TiO₂ Photocatalyst Obtained by Microwave Assisted Synthesis for the Degradation of Rhodamine B
Abstract
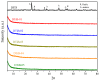
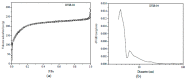
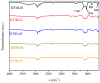
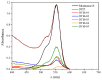
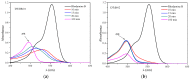
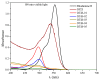
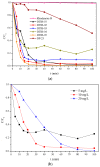
The search for novel materials and the development of improved processes for water purification have attracted the interest of researchers worldwide and the use of titanium dioxide in photocatalytic processes for the degradation of organic pollutants contained in water has been one of the benchmarks. Compared to crystalline titanium dioxide (cTiO₂), the amorphous material has the advantages of having a higher adsorption capacity and being easier to dope with metal and non-metal elements. In this work, we take advantage of these two features to improve its photocatalytic properties in the degradation of Rhodamine B. The structural characterization by XRD analysis gives evidence of its amorphous nature and the SEM micrographs portray the disc morphology of 300 nm in diameter with heterogeneous grain boundaries. The degradation of Rhodamine B tests with the amorphous TiO₂ using visible light confirm its improved catalytic activity compared to that of a commercial product, Degussa P25, which is a well-known crystalline material.
Keywords: Triple-E photocatalyst; amorphous titanium oxide; microwave assisted synthesis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Ramos-Delgado N.A., Gracia-Pinilla M.A., Mangalaraja R.V., O’Shea K., Dionysiou D.D. Industrial synthesis and characterization of nanophotocatalysts materials: Titania. Nanotechnol. Rev. 2016;5:467–479. doi: 10.1515/ntrev-2016-0007. - DOI
-
- Low J., Cheng B., Yu J. Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: A review. Appl. Surf. Sci. 2017;392:658–686. doi: 10.1016/j.apsusc.2016.09.093. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

