Advances in Brain Tumor Surgery for Glioblastoma in Adults
- PMID: 29261148
- PMCID: PMC5742769
- DOI: 10.3390/brainsci7120166
Advances in Brain Tumor Surgery for Glioblastoma in Adults
Abstract
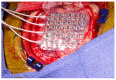
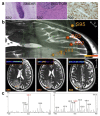
Glioblastoma (GBM) is the most common primary intracranial neoplasia, and is characterized by its extremely poor prognosis. Despite maximum surgery, chemotherapy, and radiation, the histological heterogeneity of GBM makes total eradication impossible, due to residual cancer cells invading the parenchyma, which is not otherwise seen in radiographic images. Even with gross total resection, the heterogeneity and the dormant nature of brain tumor initiating cells allow for therapeutic evasion, contributing to its recurrence and malignant progression, and severely impacting survival. Visual delimitation of the tumor's margins with common surgical techniques is a challenge faced by many surgeons. In an attempt to achieve optimal safe resection, advances in approaches allowing intraoperative analysis of cancer and non-cancer tissue have been developed and applied in humans resulting in improved outcomes. In addition, functional paradigms based on stimulation techniques to map the brain's electrical activity have optimized glioma resection in eloquent areas such as the Broca's, Wernike's and perirolandic areas. In this review, we will elaborate on the current standard therapy for newly diagnosed and recurrent glioblastoma with a focus on surgical approaches. We will describe current technologies used for glioma resection, such as awake craniotomy, fluorescence guided surgery, laser interstitial thermal therapy and intraoperative mass spectrometry. Additionally, we will describe a newly developed tool that has shown promising results in preclinical experiments for brain cancer: optical coherence tomography.
Keywords: awake craniotomy; brain tumor surgery; laser therapy; novel treatments for glioma; optical coherence tomography.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
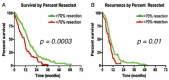
- Lacroix M., Abi-Said D., Fourney D.R., Gokaslan Z.L., Shi W., DeMonte F., Lang F.F., McCutcheon I.E., Hassenbusch S.J., Holland E., et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J. Neurosurg. 2001;95:190–198. doi: 10.3171/jns.2001.95.2.0190. - DOI - PubMed
-
- Laws E.R., Parney I.F., Huang W., Anderson F., Morris A.M., Asher A., Lillehei K.O., Bernstein M., Brem H., Sloan A., et al. Survival following surgery and prognostic factors for recently diagnosed malignant glioma: Data from the glioma outcomes project. J. Neurosurg. 2003;99:467–473. doi: 10.3171/jns.2003.99.3.0467. - DOI - PubMed
-
- Stupp R., Mason W.P., van den Bent M.J., Weller M., Fisher B., Taphoorn M.J., Belanger K., Brandes A.A., Marosi C., Bogdahn U., et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005;352:987–996. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

