Structure of the mechanically activated ion channel Piezo1
- PMID: 29261642
- PMCID: PMC6010196
- DOI: 10.1038/nature25453
Structure of the mechanically activated ion channel Piezo1
Erratum in
-
Author Correction: Structure of the mechanically activated ion channel Piezo1.Nature. 2022 Jul;607(7919):E10. doi: 10.1038/s41586-022-04976-8. Nature. 2022. PMID: 35788193 No abstract available.
Abstract
Piezo1 and Piezo2 are mechanically activated ion channels that mediate touch perception, proprioception and vascular development. Piezo proteins are distinct from other ion channels and their structure remains poorly defined, which impedes detailed study of their gating and ion permeation properties. Here we report a high-resolution cryo-electron microscopy structure of the mouse Piezo1 trimer. The detergent-solubilized complex adopts a three-bladed propeller shape with a curved transmembrane region containing at least 26 transmembrane helices per protomer. The flexible propeller blades can adopt distinct conformations, and consist of a series of four-transmembrane helical bundles that we term Piezo repeats. Carboxy-terminal domains line the central ion pore, and the channel is closed by constrictions in the cytosol. A kinked helical beam and anchor domain link the Piezo repeats to the pore, and are poised to control gating allosterically. The structure provides a foundation to dissect further how Piezo channels are regulated by mechanical force.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
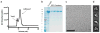
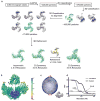



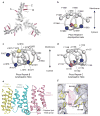





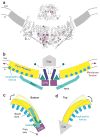
Comment in
-
Force-activated ion channels in close-up.Nature. 2018 Feb 22;554(7693):469-470. doi: 10.1038/d41586-018-01631-z. Nature. 2018. PMID: 29469134 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

