Decreased expression of lethal giant larvae causes ovarian follicle cell outgrowth in the Drosophila Scutoid mutant
- PMID: 29261681
- PMCID: PMC5737974
- DOI: 10.1371/journal.pone.0188917
Decreased expression of lethal giant larvae causes ovarian follicle cell outgrowth in the Drosophila Scutoid mutant
Abstract
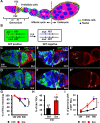
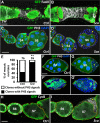
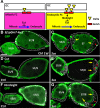
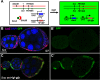
Snail, a zinc-finger transcription factor, controls the process of epithelial-mesenchymal transition, and ectopic expression of this protein may produce cells with stem cell properties. Because the effect of Snail expression in ovarian epithelial cells remains unclear, we generated Drosophila ovarian follicle stem cells (FSCs) with homozygous Scutoid (Sco) mutation. The Sco mutation is a reciprocal transposition that is known to induce ectopic Snail activity. We found that Sco mutant FSCs showed excess proliferation and high competitiveness for niche occupancy, and the descendants of this lineage formed outgrowths that failed to enter the endocycle. Surprisingly, such phenotypes were not rescued by suppressing Snail expression, but were completely restored by supplying Lethal giant larvae (Lgl). The lgl allele is a cell polarity gene that is often mutated in the genome. Importantly, Sco mutants survived in a complementation test with lgl. This result was probably obtained because the Sco-associated lgl allele appears to diminish, but not ablate lgl expression. While our data do not rule out the possibility that the Sco mutation disrupts a regulator of lgl transcription, our results strongly suggest that the phenotypes we found in Sco mutants are more closely associated with the lgl allele than ectopic Snail activity.
Conflict of interest statement
Figures


Similar articles
-
Snail controls proliferation of Drosophila ovarian epithelial follicle stem cells, independently of E-cadherin.Dev Biol. 2016 Jun 15;414(2):142-8. doi: 10.1016/j.ydbio.2016.04.022. Epub 2016 Apr 30. Dev Biol. 2016. PMID: 27141871
-
Basolateral junction proteins regulate competition for the follicle stem cell niche in the Drosophila ovary.PLoS One. 2014 Jul 3;9(7):e101085. doi: 10.1371/journal.pone.0101085. eCollection 2014. PLoS One. 2014. PMID: 24991805 Free PMC article.
-
Requirements of Lgl in cell differentiation and motility during Drosophila ovarian follicular epithelium morphogenesis.Fly (Austin). 2011 Apr-Jun;5(2):81-7. doi: 10.4161/fly.5.2.14436. Epub 2011 Apr 1. Fly (Austin). 2011. PMID: 21245664
-
Connecting epithelial polarity, proliferation and cancer in Drosophila: the many faces of lgl loss of function.Int J Dev Biol. 2013;57(9-10):677-87. doi: 10.1387/ijdb.130285dg. Int J Dev Biol. 2013. PMID: 24395559 Review.
-
Lethal giant puzzle of Lgl.Dev Neurosci. 2006;28(1-2):13-24. doi: 10.1159/000090749. Dev Neurosci. 2006. PMID: 16508300 Review.
References
-
- Kalluri R, Weinberg RA. The basics of epithelial-mesenchymal transition. J Clin Invest. 2009;119(6):1420–8. doi: 10.1172/JCI39104 ; PubMed Central PMCID: PMCPMC2689101. - DOI - PMC - PubMed
-
- Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol. 2000;2(2):76–83. doi: 10.1038/35000025 . - DOI - PubMed
-
- Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, et al. Epithelial-mesenchymal transition (EMT) induced by TNF-alpha requires AKT/GSK-3beta-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8(2):e56664 doi: 10.1371/journal.pone.0056664 ; PubMed Central PMCID: PMCPMC3576347. - DOI - PMC - PubMed
-
- Masui T, Ota I, Yook JI, Mikami S, Yane K, Yamanaka T, et al. Snail-induced epithelial-mesenchymal transition promotes cancer stem cell-like phenotype in head and neck cancer cells. Int J Oncol. 2014;44(3):693–9. doi: 10.3892/ijo.2013.2225 . - DOI - PubMed
-
- Zhou W, Lv R, Qi W, Wu D, Xu Y, Liu W, et al. Snail contributes to the maintenance of stem cell-like phenotype cells in human pancreatic cancer. PLoS One. 2014;9(1):e87409 doi: 10.1371/journal.pone.0087409 ; PubMed Central PMCID: PMCPMC3906155. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

