Estimating outflow facility through pressure dependent pathways of the human eye
- PMID: 29261696
- PMCID: PMC5738051
- DOI: 10.1371/journal.pone.0188769
Estimating outflow facility through pressure dependent pathways of the human eye
Abstract
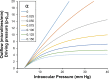
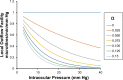
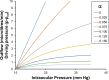
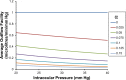
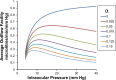
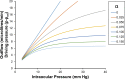
We develop and test a new theory for pressure dependent outflow from the eye. The theory comprises three main parameters: (i) a constant hydraulic conductivity, (ii) an exponential decay constant and (iii) a no-flow intraocular pressure, from which the total pressure dependent outflow, average outflow facilities and local outflow facilities for the whole eye may be evaluated. We use a new notation to specify precisely the meaning of model parameters and so model outputs. Drawing on a range of published data, we apply the theory to animal eyes, enucleated eyes and in vivo human eyes, and demonstrate how to evaluate model parameters. It is shown that the theory can fit high quality experimental data remarkably well. The new theory predicts that outflow facilities and total pressure dependent outflow for the whole eye are more than twice as large as estimates based on the Goldman equation and fluorometric analysis of anterior aqueous outflow. It appears likely that this discrepancy can be largely explained by pseudofacility and aqueous flow through the retinal pigmented epithelium, while any residual discrepancy may be due to pathological processes in aged eyes. The model predicts that if the hydraulic conductivity is too small, or the exponential decay constant is too large, then intraocular eye pressure may become unstable when subjected to normal circadian changes in aqueous production. The model also predicts relationships between variables that may be helpful when planning future experiments, and the model generates many novel testable hypotheses. With additional research, the analysis described here may find application in the differential diagnosis, prognosis and monitoring of glaucoma.
Conflict of interest statement
Figures

Similar articles
-
Estimating outflow facility parameters for the human eye using hypotensive pressure-time data.PLoS One. 2020 Aug 25;15(8):e0238146. doi: 10.1371/journal.pone.0238146. eCollection 2020. PLoS One. 2020. PMID: 32841295 Free PMC article.
-
Measurement of Outflow Facility Using iPerfusion.PLoS One. 2016 Mar 7;11(3):e0150694. doi: 10.1371/journal.pone.0150694. eCollection 2016. PLoS One. 2016. PMID: 26949939 Free PMC article.
-
Circadian variation of aqueous humor dynamics in older healthy adults.Invest Ophthalmol Vis Sci. 2013 Nov 15;54(12):7623-9. doi: 10.1167/iovs.12-12690. Invest Ophthalmol Vis Sci. 2013. PMID: 24243986 Free PMC article.
-
The effect of intraocular pressure on resistance to outflow.Surv Ophthalmol. 1977 Sep-Oct;22(2):88-100. doi: 10.1016/0039-6257(77)90088-1. Surv Ophthalmol. 1977. PMID: 335549 Review.
-
[Eye-pressure tonometry in prevention of glaucoma].Ophthalmologe. 1993 Dec;90(6):557-62. Ophthalmologe. 1993. PMID: 8124012 Review. German.
Cited by
-
New Perspective on Aqueous Humor Circulation: Retina Takes the Lead.Int J Mol Sci. 2025 Mar 14;26(6):2645. doi: 10.3390/ijms26062645. Int J Mol Sci. 2025. PMID: 40141285 Free PMC article. Review.
-
Measurement of vitreous humor pressure in vivo using an optic fiber pressure sensor.Sci Rep. 2023 Oct 25;13(1):18233. doi: 10.1038/s41598-023-45616-z. Sci Rep. 2023. PMID: 37880357 Free PMC article.
-
Estimating three-dimensional outflow and pressure gradients within the human eye.PLoS One. 2019 Apr 9;14(4):e0214961. doi: 10.1371/journal.pone.0214961. eCollection 2019. PLoS One. 2019. PMID: 30964894 Free PMC article.
-
Estimating outflow facility parameters for the human eye using hypotensive pressure-time data.PLoS One. 2020 Aug 25;15(8):e0238146. doi: 10.1371/journal.pone.0238146. eCollection 2020. PLoS One. 2020. PMID: 32841295 Free PMC article.
References
-
- Weinreb RN, Aung T, Medeiros FA. The Pathophysiology and Treatment of Glaucoma A Review. Jama-J Am Med Assoc. 2014;311(18):1901–11. doi: 10.1001/jama.2014.3192 PubMed PMID: WOS:000335798100022. - DOI - PMC - PubMed
-
- Morgan WH, Balaratnasingam C, Yu DY. The Role of Cerebrospinal Fluid Pressure in Glaucoma Pathophysiology: The Dark Side of the Optic Disc Response. J Glaucoma. 2009;18(2):172–. PubMed PMID: WOS:000263738800017. doi: 10.1097/IJG.0b013e31819aa4f9 - DOI - PubMed
-
- Berdahl JP, Yu DY, Morgan WH. The translaminar pressure gradient in sustained zero gravity, idiopathic intracranial hypertension, and glaucoma. Med Hypotheses. 2012;79(6):719–24. doi: 10.1016/j.mehy.2012.08.009 PubMed PMID: WOS:000311773500005. - DOI - PubMed
-
- Band LR, Hall CL, Richardson G, Jensen OE, Siggers JH, Foss AJE. Intracellular Flow in Optic Nerve Axons: A Mechanism for Cell Death in Glaucoma. Invest Ophth Vis Sci. 2009;50(8):3750–8. doi: 10.1167/iovs.08-2396 PubMed PMID: WOS:000268398000028. - DOI - PubMed
-
- Balaratnasingam C, Morgan WH, Bass L, Matich G, Cringle SJ, Yu DY. Axonal transport and cytoskeletal changes in the laminar regions after elevated intraocular pressure. Invest Ophth Vis Sci. 2007;48(8):3632–44. doi: 10.1167/iovs.06-1002 PubMed PMID: WOS:000248722600027. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

