Microbial cells can cooperate to resist high-level chronic ionizing radiation
- PMID: 29261697
- PMCID: PMC5738026
- DOI: 10.1371/journal.pone.0189261
Microbial cells can cooperate to resist high-level chronic ionizing radiation
Abstract
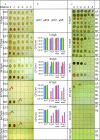
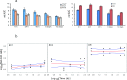
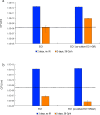
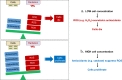
Understanding chronic ionizing radiation (CIR) effects is of utmost importance to protecting human health and the environment. Diverse bacteria and fungi inhabiting extremely radioactive waste and disaster sites (e.g. Hanford, Chernobyl, Fukushima) represent new targets of CIR research. We show that many microorganisms can grow under intense gamma-CIR dose rates of 13-126 Gy/h, with fungi identified as a particularly CIR-resistant group of eukaryotes: among 145 phylogenetically diverse strains tested, 78 grew under 36 Gy/h. Importantly, we demonstrate that CIR resistance can depend on cell concentration and that certain resistant microbial cells protect their neighbors (not only conspecifics, but even radiosensitive species from a different phylum), from high-level CIR. We apply a mechanistically-motivated mathematical model of CIR effects, based on accumulation/removal kinetics of reactive oxygen species (ROS) and antioxidants, in bacteria (3 Escherichia coli strains and Deinococcus radiodurans) and in fungi (Candida parapsilosis, Kazachstania exigua, Pichia kudriavzevii, Rhodotorula lysinophila, Saccharomyces cerevisiae, and Trichosporon mucoides). We also show that correlations between responses to CIR and acute ionizing radiation (AIR) among studied microorganisms are weak. For example, in D. radiodurans, the best molecular correlate for CIR resistance is the antioxidant enzyme catalase, which is dispensable for AIR resistance; and numerous CIR-resistant fungi are not AIR-resistant. Our experimental findings and quantitative modeling thus demonstrate the importance of investigating CIR responses directly, rather than extrapolating from AIR. Protection of radiosensitive cell-types by radioresistant ones under high-level CIR is a potentially important new tool for bioremediation of radioactive sites and development of CIR-resistant microbiota as radioprotectors.
Conflict of interest statement
Figures



References
-
- Fredrickson JK, Zachara JM, Balkwill DL, Kennedy D, Li SW, Kostandarithes HM, et al. Geomicrobiology of high-level nuclear waste-contaminated vadose sediments at the hanford site, washington state. Appl Environ Microbiol. 2004;70: 4230–41. doi: 10.1128/AEM.70.7.4230-4241.2004 - DOI - PMC - PubMed
-
- Perdrial N, Thompson A, LaSharr K, Amistadi MK, Chorover J. Quantifying Particulate and Colloidal Release of Radionuclides in Waste-Weathered Hanford Sediments. J Environ Qual. 2015;44: 945 doi: 10.2134/jeq2014.09.0388 - DOI - PubMed
-
- Kim M-HY, Rusek A, Cucinotta FA. Issues for Simulation of Galactic Cosmic Ray Exposures for Radiobiological Research at Ground-Based Accelerators. Front Oncol. 2015;5: 122 doi: 10.3389/fonc.2015.00122 - DOI - PMC - PubMed
-
- Cucinotta FA, Cacao E. Non-Targeted Effects Models Predict Significantly Higher Mars Mission Cancer Risk than Targeted Effects Models. Sci Rep. 2017;7: 1832 doi: 10.1038/s41598-017-02087-3 - DOI - PMC - PubMed
-
- Daly MJ, Gaidamakova EK, Matrosova VY, Vasilenko A, Zhai M, Venkateswaran A, et al. Accumulation of Mn(II) in Deinococcus radiodurans facilitates gamma-radiation resistance. Science. 2004;306: 1025–8. doi: 10.1126/science.1103185 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

