Shifting the boundaries for early caffeine initiation in neonatal practice: Results of a prospective, multicenter study on very preterm infants with respiratory distress syndrome
- PMID: 29261723
- PMCID: PMC5738066
- DOI: 10.1371/journal.pone.0189152
Shifting the boundaries for early caffeine initiation in neonatal practice: Results of a prospective, multicenter study on very preterm infants with respiratory distress syndrome
Abstract
Background: There is growing evidence that supports the benefits of early use of caffeine in preterm neonates with RDS; however, no formal recommendations specifying the exact timing of therapy initiation have been provided.
Objectives: We compared neonatal outcomes in infants receiving early (initial dose on the 1st day of life) and late (initial dose on day 2+ of life) caffeine therapy.
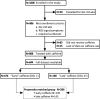
Methods: Using data from a prospective, cohort study, we identified 986 infants ≤32 weeks' gestation with RDS and assessed the timing of caffeine therapy initiation, need for ventilatory support, mortality and incidence of typical complications of prematurity. To adjust for baseline severity, the early and late caffeine groups were propensity score (PS) matched to 286 infants (1:1). Clinical outcomes were compared between the PS-matched groups.
Results: Early treatment with caffeine citrate was associated with a significantly reduced need for invasive ventilation (71.3% vs 83.2%; P = 0.0165) and total duration of mechanical ventilation (mean 5 ± 11.1 days vs 10.8 ± 14.6 days; P = 0.0000) and significantly lower odds of intraventricular hemorrhage (IVH) (OR 0.4827; 95% CI 0.2999-0.7787) and patent ductus arteriosus (PDA) (OR 0.5686; 95% CI 0.3395-0.9523). The incidence of bronchopulmonary dysplasia (BPD) (36.4% vs 45.8%) and rates of moderate and severe BPD were not significantly different between the two groups. The mortality rates were comparable between the two groups (8.6% vs 8.5%, P = ns).
Conclusion: Early caffeine initiation was associated with a decreased need for invasive ventilatory support and lower incidence of IVH and PDA.
Conflict of interest statement
Figures
Similar articles
-
Impact of early caffeine administration on respiratory outcomes in very preterm infants initially receiving invasive mechanical ventilation.BMJ Open Respir Res. 2024 Aug 28;11(1):e002285. doi: 10.1136/bmjresp-2023-002285. BMJ Open Respir Res. 2024. PMID: 39209350 Free PMC article.
-
Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants.J Pediatr. 2014 May;164(5):992-998.e3. doi: 10.1016/j.jpeds.2013.12.025. Epub 2014 Jan 23. J Pediatr. 2014. PMID: 24461786 Free PMC article.
-
Early caffeine therapy and clinical outcomes in extremely preterm infants.J Perinatol. 2013 Feb;33(2):134-40. doi: 10.1038/jp.2012.52. Epub 2012 Apr 26. J Perinatol. 2013. PMID: 22538326
-
Inhalation or instillation of steroids for the prevention of bronchopulmonary dysplasia.Neonatology. 2015;107(4):358-9. doi: 10.1159/000381132. Epub 2015 Jun 5. Neonatology. 2015. PMID: 26044104 Review.
-
A comprehensive approach to the prevention of bronchopulmonary dysplasia.Pediatr Pulmonol. 2011 Dec;46(12):1153-65. doi: 10.1002/ppul.21508. Epub 2011 Aug 3. Pediatr Pulmonol. 2011. PMID: 21815280 Review.
Cited by
-
Early caffeine therapy decreases bronchopulmonary dysplasia but might increase mortality in preterm infants? a systematic review and meta-analysis.Front Pediatr. 2025 Feb 21;13:1528054. doi: 10.3389/fped.2025.1528054. eCollection 2025. Front Pediatr. 2025. PMID: 40061428 Free PMC article.
-
Implementation of less invasive surfactant administration in clinical practice-Experience of a mid-sized country.PLoS One. 2020 Jul 6;15(7):e0235363. doi: 10.1371/journal.pone.0235363. eCollection 2020. PLoS One. 2020. PMID: 32628732 Free PMC article.
-
Confounding biases in studies on early- versus late-caffeine in preterm infants: a systematic review.Pediatr Res. 2020 Sep;88(3):357-364. doi: 10.1038/s41390-020-0757-1. Epub 2020 Jan 13. Pediatr Res. 2020. PMID: 31931506
-
Dose-Response Study of Caffeine on Postnatal Weight Gain in Premature Neonates-A Retrospective Cohort Study.Dose Response. 2024 Apr 13;22(2):15593258241247185. doi: 10.1177/15593258241247185. eCollection 2024 Apr-Jun. Dose Response. 2024. PMID: 38617389 Free PMC article.
-
A two-center retrospective study: association of early caffeine administration and oxygen radical diseases in neonatology in Chinese preterm neonates.Front Pediatr. 2023 Jun 14;11:1158286. doi: 10.3389/fped.2023.1158286. eCollection 2023. Front Pediatr. 2023. PMID: 37388282 Free PMC article.
References
-
- Park HW, Lim G, Chung SH, Chung S, Kim KS, Kim SN. Early Caffeine Use in Very Low Birth Weight Infants and Neonatal Outcomes: A Systematic Review and Meta-Analysis. J Korean Med Sci. 2015. December;30(12):1828–35. doi: 10.3346/jkms.2015.30.12.1828 - DOI - PMC - PubMed
-
- Schmidt B, Roberts RS, Davis P, Doyle LW, Barrington KJ, Ohlsson A, et al. Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N Engl J Med. 2006. May 18;354(20):2112–21. doi: 10.1056/NEJMoa054065 - DOI - PubMed
-
- Morton SU, Smith VC. Treatment options for apnoea of prematurity. Arch Dis Child Fetal Neonatal Ed. 2016. July;101(4):F352–6 doi: 10.1136/archdischild-2015-310228 - DOI - PubMed
-
- Dobson NR, Patel RM, Smith PB, Kuehn DR, Clark J, Vyas-Read S, et al. Trends in caffeine use and association between clinical outcomes and timing of therapy in very low birth weight infants. J Pediatr. 2014. May;164(5):992–998. Erratum in: J Pediatr. 2014 May;164(5):1244. doi: 10.1016/j.jpeds.2013.12.025 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous