High-dose interleukin 2 in patients with metastatic renal cell carcinoma with sarcomatoid features
- PMID: 29261796
- PMCID: PMC5738119
- DOI: 10.1371/journal.pone.0190084
High-dose interleukin 2 in patients with metastatic renal cell carcinoma with sarcomatoid features
Abstract
Background: High-dose interleukin-2 (HD IL-2) is used in the treatment of metastatic renal cell carcinoma (mRCC) and has an overall response rate (ORR) of 12-20% and a complete response rate (CR) of 8% in unselected populations with predominantly clear cell type renal cell carcinoma. Nearly 10-15% of patients with renal cell carcinoma exhibit sarcomatoid differentiation, a feature which correlates with a median overall survival (OS) of 9 months and overall poor prognosis. We report a single institution experience with 21 patients with mRCC with sarcomatoid features post-nephrectomy who were treated with HD IL-2.
Methods: Twenty one patients with mRCC with sarcomatoid features post-nephrectomy who underwent therapy with HD IL-2 were identified at the University of Pittsburgh Medical Center from 2004 to 2016. Baseline patient characteristics, HD IL-2 cycles, time to progression, and subsequent therapies were evaluated. OS and progression-free survival (PFS) in the cohort were calculated using the Kaplan-Meier method. Disease characteristics were evaluated for significance using the Fischer's exact test and Wilcoxon rank sum test.
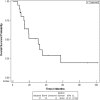
Results: Patients were predominantly Caucasian males with a median age of 54 years. A majority, 86% of these patients, had metastatic disease at time of initial presentation, primarily with lung and lymph node involvement. The ORR and CR with HD IL-2 was 10% and 5%, respectively. Initial localized disease presentation is the only variable that was significantly associated with response to HD IL-2 (p = 0.0158). Number of HD IL-2 doses did not correlate with response with a mean of 16.5 and 15.0 total doses in responders and non-responders, respectively (p = 0.53). Median PFS with HD IL-2 was 7.9 months (95% CI, 5.0-21.3). Median OS was 30.5 months (95% CI 13.3-57.66). Within the subset of patients who had progression on IL-2, median OS was 19.4 months (95% CI, 13.3-35.3). In patients who received second-line therapy, median PFS was 7.9 months (95% CI 2.4-10.2).
Conclusion: In patients with mRCC with sarcomatoid features, use of HD IL-2 was associated with a modest ORR and a higher survival compared to historical controls (patients with mRCC and sarcomatoid features). Thus, HD IL-2 may have a role in treating selected patients with mRCC with sarcomatoid features.
Conflict of interest statement
Figures
References
-
- Cangiano T, Liao J, Naitoh J, Dorey F, Figlin R, Belldegrun A. Sarcomatoid renal cell carcinoma: biologic behavior, prognosis, and response to combined surgical resection and immunotherapy. J Clin Oncol. 1999;17(2):523–8. doi: 10.1200/JCO.1999.17.2.523 . - DOI - PubMed
-
- Shuch B, Bratslavsky G, Linehan WM, Srinivasan R. Sarcomatoid renal cell carcinoma: a comprehensive review of the biology and current treatment strategies. Oncologist. 2012;17(1):46–54. doi: 10.1634/theoncologist.2011-0227 . - DOI - PMC - PubMed
-
- Golshayan AR, George S, Heng DY, Elson P, Wood LS, Mekhail TM, et al. Metastatic sarcomatoid renal cell carcinoma treated with vascular endothelial growth factor-targeted therapy. J Clin Oncol. 2009;27(2):235–41. doi: 10.1200/JCO.2008.18.0000 . - DOI - PubMed
-
- Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–96. doi: 10.1200/JCO.1995.13.3.688 . - DOI - PubMed
-
- Fyfe GA, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Long-term response data for 255 patients with metastatic renal cell carcinoma treated with high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1996;14(8):2410–1. doi: 10.1200/JCO.1996.14.8.2410 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical