Unique signalling connectivity of FGFR3-TACC3 oncoprotein revealed by quantitative phosphoproteomics and differential network analysis
- PMID: 29262532
- PMCID: PMC5732698
- DOI: 10.18632/oncotarget.22048
Unique signalling connectivity of FGFR3-TACC3 oncoprotein revealed by quantitative phosphoproteomics and differential network analysis
Abstract
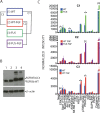
The FGFR3-TACC3 fusion is an oncogenic driver in diverse malignancies, including bladder cancer, characterized by upregulated tyrosine kinase activity. To gain insights into distinct properties of FGFR3-TACC3 down-stream signalling, we utilised telomerase-immortalised normal human urothelial cell lines expressing either the fusion or wild-type FGFR3 (isoform IIIb) for subsequent quantitative proteomics and network analysis. Cellular lysates were chemically labelled with isobaric tandem mass tag reagents and, after phosphopeptide enrichment, liquid chromatography-high mass accuracy tandem mass spectrometry (LC-MS/MS) was used for peptide identification and quantification. Comparison of data from the two cell lines under non-stimulated and FGF1 stimulated conditions and of data representing physiological stimulation of FGFR3 identified about 200 regulated phosphosites. The identified phosphoproteins and quantified phosphosites were further analysed in the context of functional biological networks by inferring kinase-substrate interactions, mapping these to a comprehensive human signalling interaction network, filtering based on tissue-expression profiles and applying disease module detection and pathway enrichment methods. Analysis of our phosphoproteomics data using these bioinformatics methods combined into a new protocol-Disease Relevant Analysis of Genes On Networks (DRAGON)-allowed us to tease apart pathways differentially involved in FGFR3-TACC3 signalling in comparison to wild-type FGFR3 and to investigate their local phospho-signalling context. We highlight 9 pathways significantly regulated only in the cell line expressing FGFR3-TACC3 fusion and 5 pathways regulated only by stimulation of the wild-type FGFR3. Pathways differentially linked to FGFR3-TACC3 fusion include those related to chaperone activation and stress response and to regulation of TP53 expression and degradation that could contribute to development and maintenance of the cancer phenotype.
Keywords: FGFR-TACC-fusion; cancer; network analysis; quantitative phosphoproteomics; signaling pathways.
Conflict of interest statement
CONFLICTS OF INTEREST There is no conflict of interest to disclose.
Figures





References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

