Xenobiotic-induced activation of human aryl hydrocarbon receptor target genes in Drosophila is mediated by the epigenetic chromatin modifiers
- PMID: 29262535
- PMCID: PMC5732701
- DOI: 10.18632/oncotarget.22173
Xenobiotic-induced activation of human aryl hydrocarbon receptor target genes in Drosophila is mediated by the epigenetic chromatin modifiers
Abstract
Aryl hydrocarbon receptor (AHR) is the key transcription factor that controls animal development and various adaptive processes. The AHR's target genes are involved in biodegradation of endogenous and exogenous toxins, regulation of immune response, organogenesis, and neurogenesis. Ligand binding is important for the activation of the AHR signaling pathway. Invertebrate AHR homologs are activated by endogenous ligands whereas vertebrate AHR can be activated by both endogenous and exogenous ligands (xenobiotics). Several studies using mammalian cultured cells have demonstrated that transcription of the AHR target genes can be activated by exogenous AHR ligands, but little is known about the effects of AHR in a living organism. Here, we examined the effects of human AHR and its ligands using transgenic Drosophila lines with an inducible human AhR gene. We found that exogenous AHR ligands can increase as well as decrease the transcription levels of the AHR target genes, including genes that control proliferation, motility, polarization, and programmed cell death. This suggests that AHR activation may affect the expression of gene networks that could be critical for cancer progression and metastasis. Importantly, we found that AHR target genes are also controlled by the enzymes that modify chromatin structure, in particular components of the epigenetic Polycomb Repressive complexes 1 and 2. Since exogenous AHR ligands (alternatively - xenobiotics) and small molecule inhibitors of epigenetic modifiers are often used as pharmaceutical anticancer drugs, our findings may have significant implications in designing new combinations of therapeutic treatments for oncological diseases.
Keywords: PcG epigenetic complexes; aryl hydrocarbon receptor; drosophila; xenobiotic.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no potential conflicts of interest.
Figures
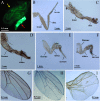
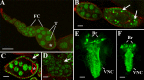



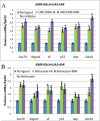
References
-
- Israel DI, Whitlock JP., Jr Induction of mRNA specific for cytochrome P1-450 in wild type and variant mouse hepatoma cells. J Biol Chem. 1983;258:10390–94. - PubMed
-
- Israel DI, Whitlock JP., Jr Regulation of cytochrome P1-450 gene transcription by 2,3,7, 8-tetrachlorodibenzo-p-dioxin in wild type and variant mouse hepatoma cells. J Biol Chem. 1984;259:5400–02. - PubMed
-
- Nebert DW, Roe AL, Dieter MZ, Solis WA, Yang Y, Dalton TP. Role of the aromatic hydrocarbon receptor and [Ah] gene battery in the oxidative stress response, cell cycle control, and apoptosis. Biochem Pharmacol. 2000;59:65–85. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

