Characterization of plasma proteins in children of different Mycobacterium tuberculosis infection status using label-free quantitative proteomics
- PMID: 29262562
- PMCID: PMC5732728
- DOI: 10.18632/oncotarget.21179
Characterization of plasma proteins in children of different Mycobacterium tuberculosis infection status using label-free quantitative proteomics
Abstract
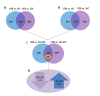
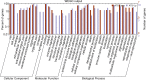
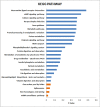
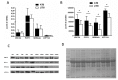
Tuberculosis (TB), caused by Mycobacterium tuberculosis (MTB), is an infectious disease found worldwide. Children infected with MTB are more likely to progress to active TB (ATB); however, the molecular mechanism behind this process has long been a mystery. We employed the label-free quantitative proteomic technology to identify and characterize differences in plasma proteins between ATB and latent TB infection (LTBI) in children. To detect differences that are indicative of MTB infection, we first selected proteins whose expressions were markedly different between the ATB and LTBI groups and the control groups (inflammatory disease control (IDC) and healthy control (HC) groups). A total of 521 proteins differed (> 1.5-fold or < 0.6-fold) in the LTBI group, and 318 proteins in the ATB group when compared with the control groups. Of these, 49 overlapping proteins were differentially expressed between LTBI and ATB. Gene Ontology (GO) analysis revealed most proteins had a cellular and organelle distribution. The MTB infection status was mainly related to differences in binding, cellular and metabolic processes. XRCC4, PCF11, SEMA4A and ATP11A were selected and further verified by qPCR and western blot. At the mRNA level, the expression of XRCC4, PCF11and SEMA4A presented an increased trend in ATB group compare with LTBI. At the protein level, the expression of all these proteins by western blot in ATB/LTBI was consistent with the trends from proteomic detection. Our results provide important data for future mechanism studies and biomarker selection for MTB infection in children.
Keywords: active tuberculosis (ATB); children; label-free quantitative proteomics; latent TB infection (LTBI); plasma proteins.
Conflict of interest statement
CONFLICTS OF INTEREST The authors have declared no conflicts of interest.
Figures

Similar articles
-
Label-Free Quantitative Proteomics Identifies Novel Plasma Biomarkers for Distinguishing Pulmonary Tuberculosis and Latent Infection.Front Microbiol. 2018 Jun 13;9:1267. doi: 10.3389/fmicb.2018.01267. eCollection 2018. Front Microbiol. 2018. PMID: 29951049 Free PMC article.
-
RNA Profiling Analysis of the Serum Exosomes Derived from Patients with Active and Latent Mycobacterium tuberculosis Infection.Front Microbiol. 2017 Jun 12;8:1051. doi: 10.3389/fmicb.2017.01051. eCollection 2017. Front Microbiol. 2017. PMID: 28659881 Free PMC article.
-
Novel serological biomarker panel using protein microarray can distinguish active TB from latent TB infection.Microbes Infect. 2022 Nov-Dec;24(8):105002. doi: 10.1016/j.micinf.2022.105002. Epub 2022 May 20. Microbes Infect. 2022. PMID: 35598729
-
[Characteristics of a diagnostic method for tuberculosis infection based on whole blood interferon-gamma assay].Kekkaku. 2006 Nov;81(11):681-6. Kekkaku. 2006. PMID: 17154047 Review. Japanese.
-
Advances in understanding immune homeostasis in latent tuberculosis infection.WIREs Mech Dis. 2024 Jul-Aug;16(4):e1643. doi: 10.1002/wsbm.1643. Epub 2024 Feb 13. WIREs Mech Dis. 2024. PMID: 38351551 Review.
Cited by
-
Aging increases the systemic molecular degree of inflammatory perturbation in patients with tuberculosis.Sci Rep. 2020 Jul 9;10(1):11358. doi: 10.1038/s41598-020-68255-0. Sci Rep. 2020. PMID: 32647178 Free PMC article.
-
The Common miRNAs between Tuberculosis and Non-Small Cell Lung Cancer: A Critical Review.Tanaffos. 2021 Mar;20(3):197-208. Tanaffos. 2021. PMID: 35382078 Free PMC article. Review.
-
Label-Free Quantitative Proteomics Identifies Novel Plasma Biomarkers for Distinguishing Pulmonary Tuberculosis and Latent Infection.Front Microbiol. 2018 Jun 13;9:1267. doi: 10.3389/fmicb.2018.01267. eCollection 2018. Front Microbiol. 2018. PMID: 29951049 Free PMC article.
-
Pediatric Tuberculosis: The Impact of "Omics" on Diagnostics Development.Int J Mol Sci. 2020 Sep 23;21(19):6979. doi: 10.3390/ijms21196979. Int J Mol Sci. 2020. PMID: 32977381 Free PMC article. Review.
-
The Low-Abundance Plasma Proteome Reveals Differentially Abundant Proteins Associated with Breast Implant Capsular Contracture: A Pilot Study.Proteomes. 2024 Aug 6;12(3):22. doi: 10.3390/proteomes12030022. Proteomes. 2024. PMID: 39189262 Free PMC article.
References
-
- Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, Wang H, Duber HC, Naghavi M, et al. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:1005–70. - PMC - PubMed
-
- Xu D, Li Y, Li X, Wei LL, Pan Z, Jiang TT, Chen ZL, Wang C, Cao WM, Zhang X, Ping ZP, Liu CM, Liu JY, et al. Serum protein S100A9, SOD3, and MMP9 as new diagnostic biomarkers for pulmonary tuberculosis by iTRAQ-coupled two-dimensional LC-MS/MS. Proteomics. 2015;15:58–67. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

