Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: involvement of the calpain / Nox1 pathway
- PMID: 29262595
- PMCID: PMC5732761
- DOI: 10.18632/oncotarget.21780
Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: involvement of the calpain / Nox1 pathway
Erratum in
-
Correction: Reversion of resistance to oxaliplatin by inhibition of p38 MAPK in colorectal cancer cell lines: involvement of the calpain / Nox1 pathway.Oncotarget. 2018 Jun 1;9(42):26978-26979. doi: 10.18632/oncotarget.25605. eCollection 2018 Jun 1. Oncotarget. 2018. PMID: 29928496 Free PMC article.
Abstract
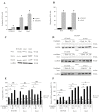
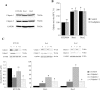
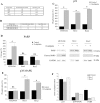
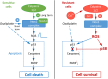
Oxaliplatin is a major treatment for metastatic colorectal cancer, however its effectiveness is greatly diminished by the development of resistances. Our previous work has shown that oxaliplatin efficacy depends on the reactive oxygen species (ROS) produced by Nox1. In this report, we investigated Nox1 involvement in the survival mechanisms of oxaliplatin resistant cell lines that we have selected. Our results show that basal ROS production by Nox1 is increased in resistant cells. Whereas the transitory Nox1-dependent production of superoxide contributes to the cytotoxicity of oxaliplatin in sensitive cells, oxaliplatin treatment of resistant cells leads to a decrease in the production of superoxide associated with an increase of H2O2 and a decreased cytotoxicity of oxaliplatin. We have shown that calpains regulate differently Nox1 according to the sensitivity of the cells to oxaliplatin. In sensitive cells, calpains inhibit Nox1 by cleaving NoxA1 leading to a transient ROS production necessary for oxaliplatin cytotoxic effects. In contrast, in resistant cells calpain activation is associated with an increase of Nox1 activity through Src kinases, inducing a strong and maintained ROS production responsible for cell survival. Using a kinomic study we have shown that this overactivation of Nox1 results in an increase of p38 MAPK activity allowing the resistant cells to escape apoptosis. Our results show that the modulation of Nox1 activity in the context of anticancer treatment remains complex. However, a strategy to maximize Nox1 activation while inhibiting the p38 MAPK-dependent escape routes appears to be an option of choice to optimize oxaliplatin efficiency.
Keywords: NADPH oxidase; calpain; chemoresistance; colorectal cancer; oxaliplatin.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures




References
-
- Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86. https://doi.org/10.1002/ijc.29210. - DOI - PubMed
-
- Van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25:iii1–9. https://doi.org/10.1093/annonc/mdu260. - DOI - PubMed
-
- Kelland L. The resurgence of platinum-based cancer chemotherapy. Nat Rev Cancer. 2007;7:573–84. https://doi.org/10.1038/nrc2167. - DOI - PubMed
-
- Bécouarn Y, Ychou M, Ducreux M, Borel C, Bertheault-Cvitkovic F, Seitz JF, Nasca S, Nguyen TD, Paillot B, Raoul JL, Duffour J, Fandi A, Dupont-André G, et al. Phase II trial of oxaliplatin as first-line chemotherapy in metastatic colorectal cancer patients. Digestive Group of French Federation of Cancer Centers. J Clin Oncol. 1998;16:2739–44. https://doi.org/10.1200/JCO.1998.16.8.2739. - DOI - PubMed
-
- Dahan L, Sadok A, Formento JL, Seitz JF, Kovacic H. Modulation of cellular redox state underlies antagonism between oxaliplatin and cetuximab in human colorectal cancer cell lines. Br J Pharmacol. 2009;158:610–20. https://doi.org/10.1111/j.1476-5381.2009.00341.x. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

