PARP inhibition causes premature loss of cohesion in cancer cells
- PMID: 29262611
- PMCID: PMC5732777
- DOI: 10.18632/oncotarget.21879
PARP inhibition causes premature loss of cohesion in cancer cells
Abstract
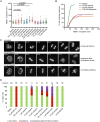
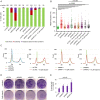
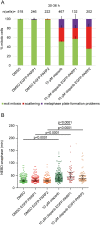
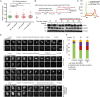
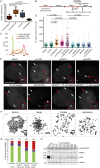
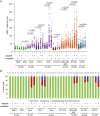
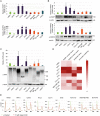
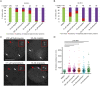
Poly(ADP-ribose) polymerases (PARPs) regulate various aspects of cellular function including mitotic progression. Although PARP inhibitors have been undergoing various clinical trials and the PARP1/2 inhibitor olaparib was approved as monotherapy for BRCA-mutated ovarian cancer, their mode of action in killing tumour cells is not fully understood. We investigated the effect of PARP inhibition on mitosis in cancerous (cervical, ovary, breast and osteosarcoma) and non-cancerous cells by live-cell imaging. The clinically relevant inhibitor olaparib induced strong perturbations in mitosis, including problems with chromosome alignment at the metaphase plate, anaphase delay, and premature loss of cohesion (cohesion fatigue) after a prolonged metaphase arrest, resulting in sister chromatid scattering. PARP1 and PARP2 depletion suppressed the phenotype while PARP2 overexpression enhanced it, suggesting that olaparib-bound PARP1 and PARP2 rather than the lack of catalytic activity causes this phenotype. Olaparib-induced mitotic chromatid scattering was observed in various cancer cell lines with increased protein levels of PARP1 and PARP2, but not in non-cancer or cancer cell lines that expressed lower levels of PARP1 or PARP2. Interestingly, the sister chromatid scattering phenotype occurred only when olaparib was added during the S-phase preceding mitosis, suggesting that PARP1 and PARP2 entrapment at replication forks impairs sister chromatid cohesion. Clinically relevant DNA-damaging agents that impair replication progression such as topoisomerase inhibitors and cisplatin were also found to induce sister chromatid scattering and metaphase plate alignment problems, suggesting that these mitotic phenotypes are a common outcome of replication perturbation.
Keywords: PARP entrapment; PARP inhibitors; cohesion; live-cell imaging; mitosis.
Conflict of interest statement
CONFLICTS OF INTEREST The authors declare no conflicts of interest.
Figures

References
-
- De Vos M, Schreiber V, Dantzer F. The diverse roles and clinical relevance of PARPs in DNA damage repair: current state of the art. Biochem Pharmacol. 2012;84:137–146. - PubMed
-
- Kraus WL, Hottiger MO. PARP-1 and gene regulation: Progress and puzzles. Mol Aspects Med. 2013;34:1109–1023. - PubMed
-
- Barkauskaite E, Jankevicius G, Ahel I. Structures and Mechanisms of Enzymes Employed in the Synthesis and Degradation of PARP-Dependent Protein ADP-Ribosylation. Mol Cell. 2015;58:935–946. - PubMed
-
- Gibson BA, Kraus WL. New insights into the molecular and cellular functions of poly(ADP-ribose) and PARPs. Nature. 2012;13:411–424. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

