Replication Rapidly Recovers and Continues in the Presence of Hydroxyurea in Escherichia coli
- PMID: 29263100
- PMCID: PMC5826035
- DOI: 10.1128/JB.00713-17
Replication Rapidly Recovers and Continues in the Presence of Hydroxyurea in Escherichia coli
Abstract
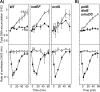
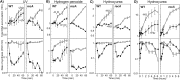
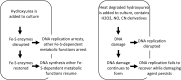
In both prokaryotes and eukaryotes, hydroxyurea is suggested to inhibit DNA replication by inactivating ribonucleotide reductase and depleting deoxyribonucleoside triphosphate pools. In this study, we show that the inhibition of replication in Escherichia coli is transient even at concentrations of 0.1 M hydroxyurea and that replication rapidly recovers and continues in its presence. The recovery of replication does not require the alternative ribonucleotide reductases NrdEF and NrdDG or the translesion DNA polymerases II (Pol II), Pol IV, and Pol V. Ribonucleotides are incorporated at higher frequencies during replication in the presence of hydroxyurea. However, they do not contribute significantly to the observed synthesis or toxicity. Hydroxyurea toxicity was observed only under conditions where the stability of hydroxyurea was compromised and by-products known to damage DNA directly were allowed to accumulate. The results demonstrate that hydroxyurea is not a direct or specific inhibitor of DNA synthesis in vivo and that the transient inhibition observed is most likely due to a general depletion of iron cofactors from enzymes when 0.1 M hydroxyurea is initially applied. Finally, the results support previous studies suggesting that hydroxyurea toxicity is mediated primarily through direct DNA damage induced by the breakdown products of hydroxyurea, rather than by inhibition of replication or depletion of deoxyribonucleotide levels in the cell.IMPORTANCE Hydroxyurea is commonly suggested to function by inhibiting DNA replication through the inactivation of ribonucleotide reductase and depleting deoxyribonucleoside triphosphate pools. Here, we show that hydroxyurea only transiently inhibits replication in Escherichia coli before replication rapidly recovers and continues in the presence of the drug. The recovery of replication does not depend on alternative ribonucleotide reductases, translesion synthesis, or RecA. Further, we show that hydroxyurea toxicity is observed only in the presence of toxic intermediates that accumulate when hydroxyurea breaks down, damage DNA, and induce lethality. The results demonstrate that hydroxyurea toxicity is mediated indirectly by the formation of DNA damage, rather than by inhibition of replication or depletion of deoxyribonucleotide levels in the cell.
Keywords: DNA replication; RNase H; hydroxyurea; translesion DNA synthesis.
Copyright © 2018 American Society for Microbiology.
Figures



References
-
- Stearns B, Losee KA, Bernstein J. 1963. Hydroxyurea. A new type of potential antitumor agent. J Med Chem 6:201. - PubMed
-
- Stauber RH, Knauer SK, Habtemichael N, Bier C, Unruhe B, Weisheit S, Spange S, Nonnenmacher F, Fetz V, Ginter T, Reichardt S, Liebmann C, Schneider G, Krämer OH. 2012. A combination of a ribonucleotide reductase inhibitor and histone deacetylase inhibitors downregulates EGFR and triggers BIM-dependent apoptosis in head and neck cancer. Oncotarget 3:31–43. doi:10.18632/oncotarget.430. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

