Immunoproteasome Subunits Are Required for CD8+ T Cell Function and Host Resistance to Brucella abortus Infection in Mice
- PMID: 29263103
- PMCID: PMC5820958
- DOI: 10.1128/IAI.00615-17
Immunoproteasome Subunits Are Required for CD8+ T Cell Function and Host Resistance to Brucella abortus Infection in Mice
Abstract
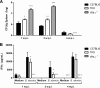
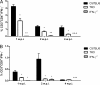
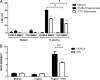
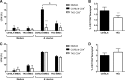
The immunoproteasome is a specific proteasome isoform composed of three subunits, termed β1i, β2i, and β5i. Its proteolytic activity enhances the quantity and quality of peptides to be presented by major histocompatibility complex class I (MHC-I) molecules to CD8+ T cells. However, the role of the combined deficiency of the three immunoproteasome subunits in protective immunity against bacterial pathogens has not been investigated. In this study, we addressed the role of the immunoproteasome during infection by Brucella abortus, an intracellular bacterium that requires CD8+ T cell responses for the control of infection. Here, we demonstrate that immunoproteasome triple-knockout (TKO) mice were more susceptible to Brucella infection. This observed susceptibility was accompanied by reduced interferon gamma (IFN-γ) production by mouse CD4+ and CD8+ T lymphocytes. Moreover, the absence of the immunoproteasome had an impact on MHC-I surface expression and antigen presentation by dendritic cells. CD8+ T cell function, which plays a pivotal role in B. abortus immunity, also presented a partial impairment of granzyme B expression and, consequently, reduced cytotoxic activity. In conclusion, these results strongly suggest that immunoproteasome subunits are important components in host resistance to B. abortus infection by impacting both the magnitude and quality of CD8+ T cell responses.
Keywords: Brucella abortus; CD8+ T cells; MHC-I; immunoproteasome.
Copyright © 2018 American Society for Microbiology.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

