Pathogenicity of a Human Laminin β 2 Mutation Revealed in Models of Alport Syndrome
- PMID: 29263159
- PMCID: PMC5827610
- DOI: 10.1681/ASN.2017090997
Pathogenicity of a Human Laminin β 2 Mutation Revealed in Models of Alport Syndrome
Abstract
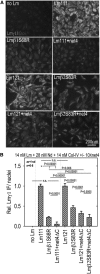
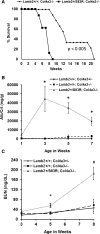
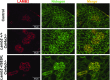
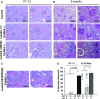
Pierson syndrome is a congenital nephrotic syndrome with eye and neurologic defects caused by mutations in laminin β2 (LAMB2), a major component of the glomerular basement membrane (GBM). Pathogenic missense mutations in human LAMB2 cluster in or near the laminin amino-terminal (LN) domain, a domain required for extracellular polymerization of laminin trimers and basement membrane scaffolding. Here, we investigated an LN domain missense mutation, LAMB2-S80R, which was discovered in a patient with Pierson syndrome and unusually late onset of proteinuria. Biochemical data indicated that this mutation impairs laminin polymerization, which we hypothesized to be the cause of the patient's nephrotic syndrome. Testing this hypothesis in genetically altered mice showed that the corresponding amino acid change (LAMB2-S83R) alone is not pathogenic. However, expression of LAMB2-S83R significantly increased the rate of progression to kidney failure in a Col4a3-/- mouse model of autosomal recessive Alport syndrome and increased proteinuria in Col4a5+/- females that exhibit a mild form of X-linked Alport syndrome due to mosaic deposition of collagen α3α4α5(IV) in the GBM. Collectively, these data show the pathogenicity of LAMB2-S80R and provide the first evidence of genetic modification of Alport phenotypes by variation in another GBM component. This finding could help explain the wide range of Alport syndrome onset and severity observed in patients with Alport syndrome, even for family members who share the same COL4 mutation. Our results also show the complexities of using model organisms to investigate genetic variants suspected of being pathogenic in humans.
Keywords: Alport syndrome; glomerular basement membrane; laminin; nephrotic syndrome.
Copyright © 2018 by the American Society of Nephrology.
Figures



References
-
- Miner JH: Renal basement membrane components. Kidney Int 56: 2016–2024, 1999 - PubMed
-
- Yurchenco PD, Amenta PS, Patton BL: Basement membrane assembly, stability and activities observed through a developmental lens. Matrix Biol 22: 521–538, 2004 - PubMed
-
- Yurchenco PD, Cheng YS: Self-assembly and calcium-binding sites in laminin. A three-arm interaction model. J Biol Chem 268: 17286–17299, 1993 - PubMed
-
- Miner JH: Building the glomerulus: A matricentric view. J Am Soc Nephrol 16: 857–861, 2005 - PubMed
-
- Yurchenco PD: Integrating activities of laminins that drive basement membrane assembly and function. Curr Top Membr 76: 1–30, 2015 - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

