Development of Envelope Protein Antigens To Serologically Differentiate Zika Virus Infection from Dengue Virus Infection
- PMID: 29263206
- PMCID: PMC5824056
- DOI: 10.1128/JCM.01504-17
Development of Envelope Protein Antigens To Serologically Differentiate Zika Virus Infection from Dengue Virus Infection
Abstract
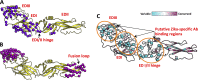
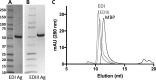
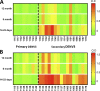
Zika virus (ZIKV) is an emerging flavivirus that can cause birth defects and neurologic complications. Molecular tests are effective for diagnosing acute ZIKV infection, although the majority of infections produce no symptoms at all or present after the narrow window in which molecular diagnostics are dependable. Serology is a reliable method for detecting infections after the viremic period; however, most serological assays have limited specificity due to cross-reactive antibodies elicited by flavivirus infections. Since ZIKV and dengue virus (DENV) widely cocirculate, distinguishing ZIKV infection from DENV infection is particularly important for diagnosing individual cases or for surveillance to coordinate public health responses. Flaviviruses also elicit type-specific antibodies directed to non-cross-reactive epitopes of the infecting virus; such epitopes are attractive targets for the design of antigens for development of serological tests with greater specificity. Guided by comparative epitope modeling of the ZIKV envelope protein, we designed two recombinant antigens displaying unique antigenic regions on domain I (Z-EDI) and domain III (Z-EDIII) of the ZIKV envelope protein. Both the Z-EDI and Z-EDIII antigens consistently detected ZIKV-specific IgG in ZIKV-immune sera but not cross-reactive IgG in DENV-immune sera in late convalescence (>12 weeks postinfection). In contrast, during early convalescence (2 to 12 weeks postinfection), secondary DENV-immune sera and some primary DENV-immune sera cross-reacted with the Z-EDI and Z-EDIII antigens. Analysis of sequential samples from DENV-immune individuals demonstrated that Z-EDIII cross-reactivity peaked in early convalescence and declined steeply over time. The Z-EDIII antigen has much potential as a diagnostic antigen for population-level surveillance and for detecting past infections in patients.
Keywords: ELISA; Zika virus; antibody-binding region; comparative epitope mapping; computational prediction; cross-reactivity; dengue virus; flavivirus; serological diagnosis; surveillance.
Copyright © 2018 American Society for Microbiology.
Figures


References
-
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimaraes KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandao WN, Rossato C, Andrade DG, Faria DP, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrao-Braga PC. 2016. The Brazilian Zika virus strain causes birth defects in experimental models. Nature 534:267–271. doi:10.1038/nature18296. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

