Attention Shifts Recruit the Monkey Default Mode Network
- PMID: 29263238
- PMCID: PMC6596261
- DOI: 10.1523/JNEUROSCI.1111-17.2017
Attention Shifts Recruit the Monkey Default Mode Network
Abstract
A unifying function associated with the default mode network (DMN), which is more active during rest than under active task conditions, has been difficult to define. The DMN is activated during monitoring the external world for unexpected events, as a sentinel, and when humans are engaged in high-level internally focused tasks. The existence of DMN correlates in other species, such as mice, challenge the idea that internally focused, high-level cognitive operations, such as introspection, autobiographical memory retrieval, planning the future, and predicting someone else's thoughts, are evolutionarily preserved defining properties of the DMN. A recent human study demonstrated that demanding cognitive shifts could recruit the DMN, yet it is unknown whether this holds for nonhuman species. Therefore, we tested whether large changes in cognitive context would recruit DMN regions in female and male nonhuman primates. Such changes were measured as displacements of spatial attentional weights based on internal rules of relevance (spatial shifts) compared with maintaining attentional weights at the same location (stay events). Using fMRI in macaques, we detected that a cortical network, activated during shifts, largely overlapped with the DMN. Moreover, fMRI time courses sampled from independently defined DMN foci showed significant shift selectivity during the demanding attention task. Finally, functional clustering based on independent resting state data revealed that DMN and shift regions clustered conjointly, whereas regions activated during the stay events clustered apart. We therefore propose that cognitive shifting in primates generally recruits DMN regions. This might explain a breakdown of the DMN in many neurological diseases characterized by declined cognitive flexibility.SIGNIFICANCE STATEMENT Activation of the human default mode network (DMN) can be measured with fMRI when subjects shift thoughts between high-level internally directed cognitive states, when thinking about the self, the perspective of others, when imagining future and past events, and during mind wandering. Furthermore, the DMN is activated as a sentinel, monitoring the environment for unexpected events. Arguably, these cognitive processes have in common fast and substantial changes in cognitive context. As DMN activity has also been reported in nonhuman species, we tested whether shifts in spatial attention activated the monkey DMN. Core monkey DMN and shift-selective regions shared several functional properties, indicating that cognitive shifting, in general, might constitute one of the evolutionarily preserved functions of the DMN.
Keywords: DMN; attention; cognition; fMRI; monkey.
Copyright © 2018 the authors 0270-6474/18/381202-16$15.00/0.
Figures
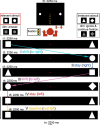
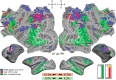
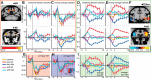
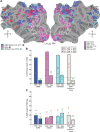


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
