Cocaine Selectively Reorganizes Excitatory Inputs to Substantia Nigra Pars Compacta Dopamine Neurons
- PMID: 29263240
- PMCID: PMC5792475
- DOI: 10.1523/JNEUROSCI.1975-17.2017
Cocaine Selectively Reorganizes Excitatory Inputs to Substantia Nigra Pars Compacta Dopamine Neurons
Abstract
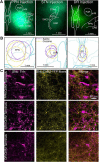
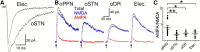
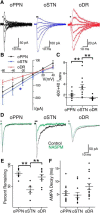
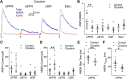
Substantia nigra pars compacta (SNc) dopamine neurons and their targets are involved in addiction and cue-induced relapse. However, afferents onto SNc dopamine neurons themselves appear insensitive to drugs of abuse, such as cocaine, when afferents are collectively stimulated electrically. This contrasts with ventral tegmental area (VTA) dopamine neurons, whose glutamate afferents react robustly to cocaine. We used an optogenetic strategy to isolate identified SNc inputs and determine whether cocaine sensitivity in the mouse SNc circuit is conferred at the level of three glutamate afferents: dorsal raphé nucleus (DR), pedunculopontine nucleus (PPN), and subthalamic nucleus (STN). We found that excitatory afferents to SNc dopamine neurons are sensitive to cocaine in an afferent-specific manner. A single exposure to cocaine in vivo led to PPN-innervated synapses reducing the AMPA-to-NMDA receptor-mediated current ratio. In contrast to work in the VTA, this was due to increased NMDA receptor function with no change in AMPA receptor function. STN synapses showed a decrease in calcium-permeable AMPA receptors after cocaine, but no change in the AMPA-to-NMDA ratio. Cocaine also increased the release probability at DR-innervated and STN-innervated synapses, quantified by decreases in paired-pulse ratios. However, release probability at PPN-innervated synapses remained unaffected. By examining identified inputs, our results demonstrate a functional distribution among excitatory SNc afferent nuclei in response to cocaine, and suggest a compelling architecture for differentiation and separate parsing of inputs within the nigrostriatal system.SIGNIFICANCE STATEMENT Prior studies have established that substantia nigra pars compacta (SNc) dopamine neurons are a key node in the circuitry that drives addiction and relapse, yet cocaine apparently has no effect on electrically stimulated excitatory inputs. Our study is the first to demonstrate the functional impact of a drug of abuse on synaptic mechanisms of identified afferents to the SNc. Optogenetic dissection of inputs originating from dorsal raphé, pedunculopontine, and subthalamic nuclei were tested for synaptic modifications following in vivo cocaine exposure. Our results demonstrate that cocaine differentially induces modifications to SNc synapses depending on input origin. This presents implications for understanding dopamine processing of motivated behavior; most critically, it indicates that dopamine neurons selectively modulate signal reception processed by afferent nuclei.
Keywords: AMPA; NMDA; dorsal raphé; optogenetics; pedunculopontine nucleus; subthalamic nucleus.
Copyright © 2018 the authors 0270-6474/18/381151-09$15.00/0.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
