Actin-Dependent Nonlytic Rotavirus Exit and Infectious Virus Morphogenetic Pathway in Nonpolarized Cells
- PMID: 29263265
- PMCID: PMC5827380
- DOI: 10.1128/JVI.02076-17
Actin-Dependent Nonlytic Rotavirus Exit and Infectious Virus Morphogenetic Pathway in Nonpolarized Cells
Abstract
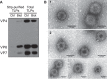
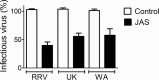
During the late stages of rotavirus morphogenesis, the surface proteins VP4 and VP7 are assembled onto the previously structured double-layered virus particles to yield a triple-layered, mature infectious virus. The current model for the assembly of the outer capsid is that it occurs within the lumen of the endoplasmic reticulum. However, it has been shown that VP4 and infectious virus associate with lipid rafts, suggesting that the final assembly of the rotavirus spike protein VP4 involves a post-endoplasmic reticulum event. In this work, we found that the actin inhibitor jasplakinolide blocks the cell egress of rotavirus from nonpolarized MA104 cells at early times of infection, when there is still no evidence of cell lysis. These findings contrast with the traditional assumption that rotavirus is released from nonpolarized cells by a nonspecific mechanism when the cell integrity is lost. Inspection of the virus present in the extracellular medium by use of density flotation gradients revealed that a fraction of the released virus is associated with low-density membranous structures. Furthermore, the intracellular localization of VP4, its interaction with lipid rafts, and its targeting to the cell surface were shown to be prevented by jasplakinolide, implying a role for actin in these processes. Finally, the VP4 present at the plasma membrane was shown to be incorporated into the extracellular infectious virus, suggesting the existence of a novel pathway for the assembly of the rotavirus spike protein.IMPORTANCE Rotavirus is a major etiological agent of infantile acute severe diarrhea. It is a nonenveloped virus formed by three concentric layers of protein. The early stages of rotavirus replication, including cell attachment and entry, synthesis and translation of viral mRNAs, replication of the genomic double-stranded RNA (dsRNA), and the assembly of double-layered viral particles, have been studied widely. However, the mechanisms involved in the later stages of infection, i.e., viral particle maturation and cell exit, are less well characterized. It has been assumed historically that rotavirus exits nonpolarized cells following cell lysis. In this work, we show that the virus exits cells by a nonlytic, actin-dependent mechanism, and most importantly, we describe that VP4, the spike protein of the virus, is present on the cell surface and is incorporated into mature, infectious virus, indicating a novel pathway for the assembly of this protein.
Keywords: cell exit; morphogenesis; rotavirus; virus assembly.
Copyright © 2018 American Society for Microbiology.
Figures
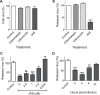
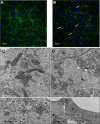

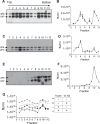
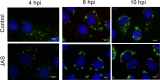



References
-
- Estes MK, Greenberg HB. 2013. Rotaviruses, p 1347–1401. In Knipe DM, Howley PM, Cohen JI, Griffin DE, Lamb RA, Martin MA, Racaniello VR, Roizman B (ed), Fields virology, 6th ed Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Li B, Ding S, Feng N, Mooney N, Ooi YS, Ren L, Diep J, Kelly MR, Yasukawa LL, Patton JT, Yamazaki H, Shirao T, Jackson PK, Greenberg HB. 2017. Drebrin restricts rotavirus entry by inhibiting dynamin-mediated endocytosis. Proc Natl Acad Sci U S A 114:E3642–E3651. doi: 10.1073/pnas.1619266114. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

