The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling
- PMID: 29263274
- PMCID: PMC5827370
- DOI: 10.1128/JVI.01737-17
The Human Papillomavirus E6 Oncoprotein Targets USP15 and TRIM25 To Suppress RIG-I-Mediated Innate Immune Signaling
Abstract
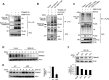
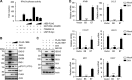
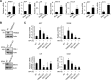
Retinoic acid-inducible gene I (RIG-I) is a key pattern recognition receptor that senses viral RNA and interacts with the mitochondrial adaptor MAVS, triggering a signaling cascade that results in the production of type I interferons (IFNs). This signaling axis is initiated by K63-linked ubiquitination of RIG-I mediated by the E3 ubiquitin ligase TRIM25, which promotes the interaction of RIG-I with MAVS. USP15 was recently identified as an upstream regulator of TRIM25, stabilizing the enzyme through removal of degradative K48-linked polyubiquitin, ultimately promoting RIG-I-dependent cytokine responses. Here, we show that the E6 oncoprotein of human papillomavirus type 16 (HPV16) as well as of other HPV types form a complex with TRIM25 and USP15 in human cells. In the presence of E6, the K48-linked ubiquitination of TRIM25 was markedly increased, and in line with this, TRIM25 degradation was enhanced. Our results further showed that E6 inhibited the TRIM25-mediated K63-linked ubiquitination of RIG-I and its CARD-dependent interaction with MAVS. HPV16 E6, but not E7, suppressed the RIG-I-mediated induction of IFN-β, chemokines, and IFN-stimulated genes (ISGs). Finally, CRISPR-Cas9 gene targeting in human keratinocytes showed that the TRIM25-RIG-I-MAVS triad is important for eliciting an antiviral immune response to HPV16 infection. Our study thus identifies a novel immune escape mechanism that is conserved among different HPV strains and further indicates that the RIG-I signaling pathway plays an important role in the innate immune response to HPV infection.IMPORTANCE Persistent infection and tumorigenesis by HPVs are known to require viral manipulation of a variety of cellular processes, including those involved in innate immune responses. Here, we show that the HPV E6 oncoprotein antagonizes the activation of the cytoplasmic innate immune sensor RIG-I by targeting its upstream regulatory enzymes TRIM25 and USP15. We further show that the RIG-I signaling cascade is important for an antiviral innate immune response to HPV16 infection, providing evidence that RIG-I, whose role in sensing RNA virus infections has been well characterized, also plays a crucial role in the antiviral host response to small DNA viruses of the Papillomaviridae family.
Keywords: RIG-I; innate immunity; interferons; papillomavirus.
Copyright © 2018 American Society for Microbiology.
Figures


References
-
- Goubau D, Schlee M, Deddouche S, Pruijssers AJ, Zillinger T, Goldeck M, Schuberth C, Van der Veen AG, Fujimura T, Rehwinkel J, Iskarpatyoti JA, Barchet W, Ludwig J, Dermody TS, Hartmann G, Reis ESC. 2014. Antiviral immunity via RIG-I-mediated recognition of RNA bearing 5′-diphosphates. Nature 514:372–375. doi: 10.1038/nature13590. - DOI - PMC - PubMed
-
- Kato H, Takeuchi O, Sato S, Yoneyama M, Yamamoto M, Matsui K, Uematsu S, Jung A, Kawai T, Ishii KJ, Yamaguchi O, Otsu K, Tsujimura T, Koh CS, Reis e Sousa C, Matsuura Y, Fujita T, Akira S. 2006. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature 441:101–105. doi: 10.1038/nature04734. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

